Abstract
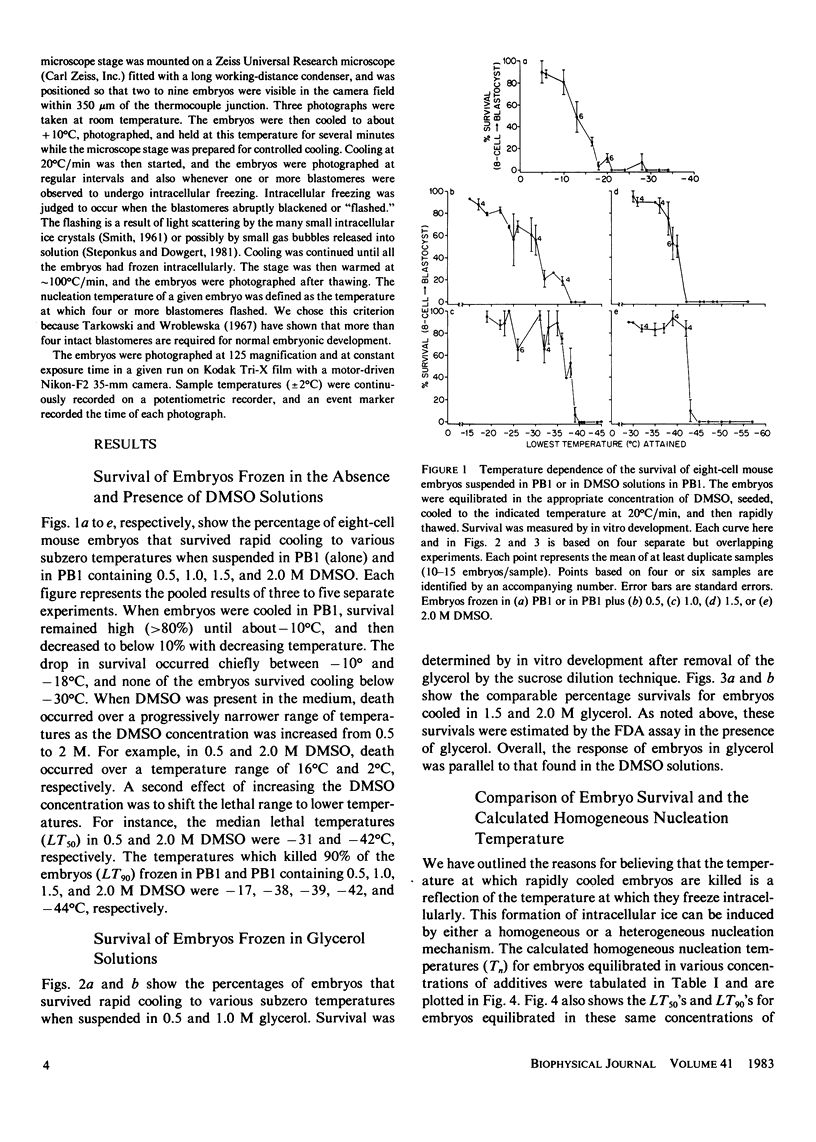
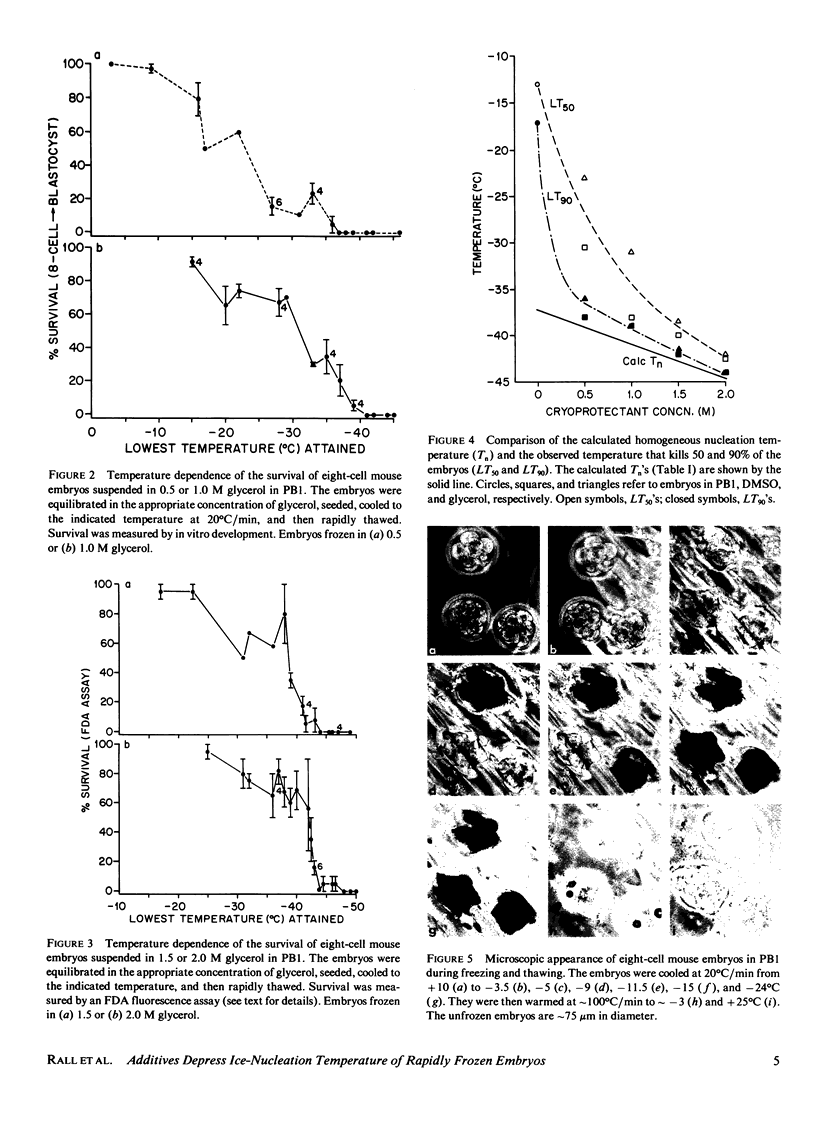
The temperature at which ice formation occurs in supercooled cytoplasm is an important element in predicting the likelihood of intracellular freezing of cells cooled by various procedures to subzero temperatures. We have confirmed and extended prior indications that permeating cryoprotective additives decrease the ice nucleation temperature of cells, and have determined some possible mechanisms for the decrease. Our experiments were carried out on eight-cell mouse embryos equilibrated with various concentrations (0-2.0 M) of dimethyl sulfoxide or glycerol and then cooled rapidly. Two methods were used to assess the nucleation temperature. The first, indirect, method was to determine the in vitro survival of the rapidly cooled embryos as a function of temperature. The temperatures over which an abrupt drop in survival occurs are generally diagnostic of the temperature range for intracellular freezing. The second, direct, method was to observe the microscopic appearance during rapid cooling and note the temperature at which nucleation occurred. Both methods showed that the nucleation temperature decreased from - 10 to - 15 degrees C in saline alone to between - 38 degrees and - 44 degrees C in 1.0-2.0 M glycerol and dimethyl sulfoxide. The latter two temperatures are close to the homogeneous nucleation temperatures of the solutions in the embryo cytoplasm, and suggest that embryos equilibrated in these solutions do not contain heterogeneous nucleating agents and are not accessible to any extracellular nucleating agents, such as extracellular ice. The much higher freezing temperatures of cells in saline or in low concentrations of additive indicate that they are being nucleated by heterogeneous agents or, more likely, by extracellular ice.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asahina E., Shimada K., Hisada Y. A stable state of frozen protoplasm with invisible intracellular ice crystals obtained by rapid cooling. Exp Cell Res. 1970 Mar;59(3):349–358. doi: 10.1016/0014-4827(70)90641-5. [DOI] [PubMed] [Google Scholar]
- BRINSTER R. L. A METHOD FOR IN VITRO CULTIVATION OF MOUSE OVA FROM TWO-CELL TO BLASTOCYST. Exp Cell Res. 1963 Oct;32:205–208. doi: 10.1016/0014-4827(63)90093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank H. Freezing injury in tissue cultured cells as visualized by freeze-etching. Exp Cell Res. 1974 Apr;85(2):367–376. doi: 10.1016/0014-4827(74)90138-4. [DOI] [PubMed] [Google Scholar]
- Diller K. R., Cravalho E. G., Huggins C. E. Intracellular freezing in biomaterials. Cryobiology. 1972 Oct;9(5):429–440. doi: 10.1016/0011-2240(72)90160-5. [DOI] [PubMed] [Google Scholar]
- Ducibella T., Anderson E. Cell shape and membrane changes in the eight-cell mouse embryo: prerequisites for morphogenesis of the blastocyst. Dev Biol. 1975 Nov;47(1):45–58. doi: 10.1016/0012-1606(75)90262-6. [DOI] [PubMed] [Google Scholar]
- Farrant J. Water transport and cell survival in cryobiological procedures. Philos Trans R Soc Lond B Biol Sci. 1977 Mar 29;278(959):191–205. doi: 10.1098/rstb.1977.0037. [DOI] [PubMed] [Google Scholar]
- Knight S. C., Farrant J., McGann L. E. Storage of human lymphocytes by freezing in serum alone. Cryobiology. 1977 Feb;14(1):112–115. doi: 10.1016/0011-2240(77)90129-8. [DOI] [PubMed] [Google Scholar]
- LOVELOCK J. E., BISHOP M. W. Prevention of freezing damage to living cells by dimethyl sulphoxide. Nature. 1959 May 16;183(4672):1394–1395. doi: 10.1038/1831394a0. [DOI] [PubMed] [Google Scholar]
- LOVELOCK J. E. The mechanism of the protective action of glycerol against haemolysis by freezing and thawing. Biochim Biophys Acta. 1953 May;11(1):28–36. doi: 10.1016/0006-3002(53)90005-5. [DOI] [PubMed] [Google Scholar]
- Leibo S. P. Fundamental cryobiology of mouse ova and embryos. Ciba Found Symp. 1977 Jan 18;(52):69–96. doi: 10.1002/9780470720332.ch5. [DOI] [PubMed] [Google Scholar]
- Leibo S. P., McGrath J. J., Cravalho E. G. Microscopic observation of intracellular ice formation in unfertilized mouse ova as a function of cooling rate. Cryobiology. 1978 Jun;15(3):257–271. doi: 10.1016/0011-2240(78)90036-6. [DOI] [PubMed] [Google Scholar]
- Leibo S. P. Water permeability and its activation energy of fertilized and unfertilized mouse ova. J Membr Biol. 1980;53(3):179–188. doi: 10.1007/BF01868823. [DOI] [PubMed] [Google Scholar]
- Luyet B., Pribor D. Direct observations of hemolysis during the rewarming and the thawing of frozen blood. Biodynamica. 1965 Nov;9(191):319–332. [PubMed] [Google Scholar]
- MAFFLY L. H., LEAF A. Water activity of mammalian tissues. Nature. 1958 Jul 5;182(4627):60–61. doi: 10.1038/182060a0. [DOI] [PubMed] [Google Scholar]
- MAZUR P. CAUSES OF INJURY IN FROZEN AND THAWED CELLS. Fed Proc. 1965 Mar-Apr;24:S175–S182. [PubMed] [Google Scholar]
- MAZUR P. KINETICS OF WATER LOSS FROM CELLS AT SUBZERO TEMPERATURES AND THE LIKELIHOOD OF INTRACELLULAR FREEZING. J Gen Physiol. 1963 Nov;47:347–369. doi: 10.1085/jgp.47.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAZUR P. Studies on rapidly frozen suspensions of yeast cells by differential thermal analysis and conductometry. Biophys J. 1963 Jul;3:323–353. doi: 10.1016/s0006-3495(63)86824-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOOR H. DIE GEFRIER-FIXATION LEBENDER ZELLEN UND IHRE ANWENDUNG IN DER ELEKTRONENMIKROSKOPIE. Z Zellforsch Mikrosk Anat. 1964 Apr 28;62:546–580. [PubMed] [Google Scholar]
- Mansoori G. A. Kinetics of water loss from cells at subzero centigrade temperatures. Cryobiology. 1975 Feb;12(1):34–45. doi: 10.1016/0011-2240(75)90039-5. [DOI] [PubMed] [Google Scholar]
- Mazur P., Rall W. F., Rigopoulos N. Relative contributions of the fraction of unfrozen water and of salt concentration to the survival of slowly frozen human erythrocytes. Biophys J. 1981 Dec;36(3):653–675. doi: 10.1016/S0006-3495(81)84757-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur P. The role of intracellular freezing in the death of cells cooled at supraoptimal rates. Cryobiology. 1977 Jun;14(3):251–272. doi: 10.1016/0011-2240(77)90175-4. [DOI] [PubMed] [Google Scholar]
- McGann L. E. Differing actions of penetrating and nonpenetrating cryoprotective agents. Cryobiology. 1978 Aug;15(4):382–390. doi: 10.1016/0011-2240(78)90056-1. [DOI] [PubMed] [Google Scholar]
- McGrath J. J., Cravalho E. G., Huggins C. E. An experimental comparison of intracellular ice formation and freeze-thaw survival of HeLa S-3 cells. Cryobiology. 1975 Dec;12(6):540–550. doi: 10.1016/0011-2240(75)90048-6. [DOI] [PubMed] [Google Scholar]
- Rall W. F., Mazur P., Souzu H. Physical-chemical basis of the protection of slowly frozen human erythrocytes by glycerol. Biophys J. 1978 Jul;23(1):101–120. doi: 10.1016/S0006-3495(78)85436-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall W. F., Reid D. S., Farrant J. Innocuous biological freezing during warming. Nature. 1980 Jul 31;286(5772):511–514. doi: 10.1038/286511a0. [DOI] [PubMed] [Google Scholar]
- Rasmussen D. H., Macaulay M. N., MacKenzie A. P. Supercooling and nucleation of ice in single cells. Cryobiology. 1975 Aug;12(4):328–339. doi: 10.1016/0011-2240(75)90006-1. [DOI] [PubMed] [Google Scholar]
- Rotman B., Papermaster B. W. Membrane properties of living mammalian cells as studied by enzymatic hydrolysis of fluorogenic esters. Proc Natl Acad Sci U S A. 1966 Jan;55(1):134–141. doi: 10.1073/pnas.55.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Asahina E. Visualization of intracellular ice crystals formed in very rapidly frozen cells at -27 degree C. Cryobiology. 1975 Jun;12(3):209–218. doi: 10.1016/0011-2240(75)90019-x. [DOI] [PubMed] [Google Scholar]
- Staehelin L. A., Bertaud W. S. Temperature and contamination dependent freeze-etch images of frozen water and glycerol solutions. J Ultrastruct Res. 1971 Oct;37(1):146–168. doi: 10.1016/s0022-5320(71)80047-3. [DOI] [PubMed] [Google Scholar]
- Tarkowski A. K., Wróblewska J. Development of blastomeres of mouse eggs isolated at the 4- and 8-cell stage. J Embryol Exp Morphol. 1967 Aug;18(1):155–180. [PubMed] [Google Scholar]
- WOOD T. H., ROSENBERG A. M. Freezing in yeast cells. Biochim Biophys Acta. 1957 Jul;25(1):78–87. doi: 10.1016/0006-3002(57)90421-3. [DOI] [PubMed] [Google Scholar]
- Whittingham D. G. Culture of mouse ova. J Reprod Fertil Suppl. 1971 Jun;14:7–21. [PubMed] [Google Scholar]
- Whittingham D. G. Embryo banks in the future of developmental genetics. Genetics. 1974 Sep;78(1):395–402. doi: 10.1093/genetics/78.1.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittingham D. G., Leibo S. P., Mazur P. Survival of mouse embryos frozen to -196 degrees and -269 degrees C. Science. 1972 Oct 27;178(4059):411–414. [PubMed] [Google Scholar]