Abstract
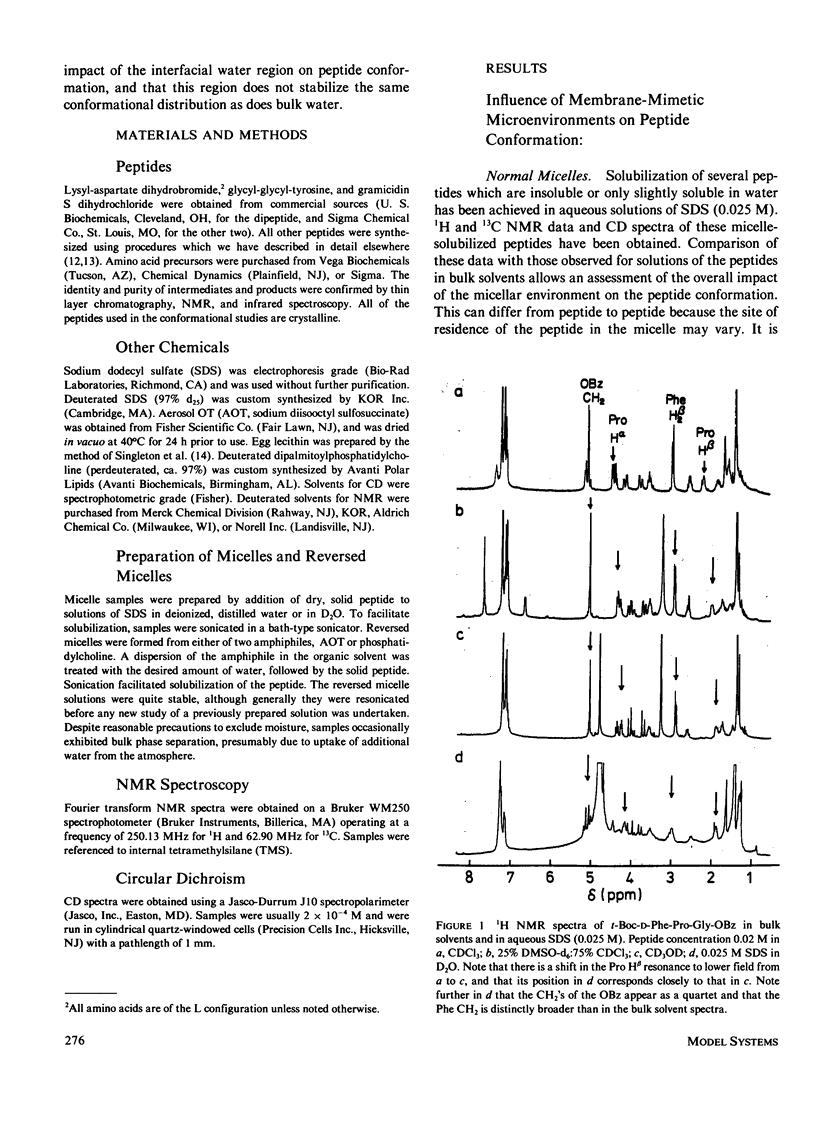
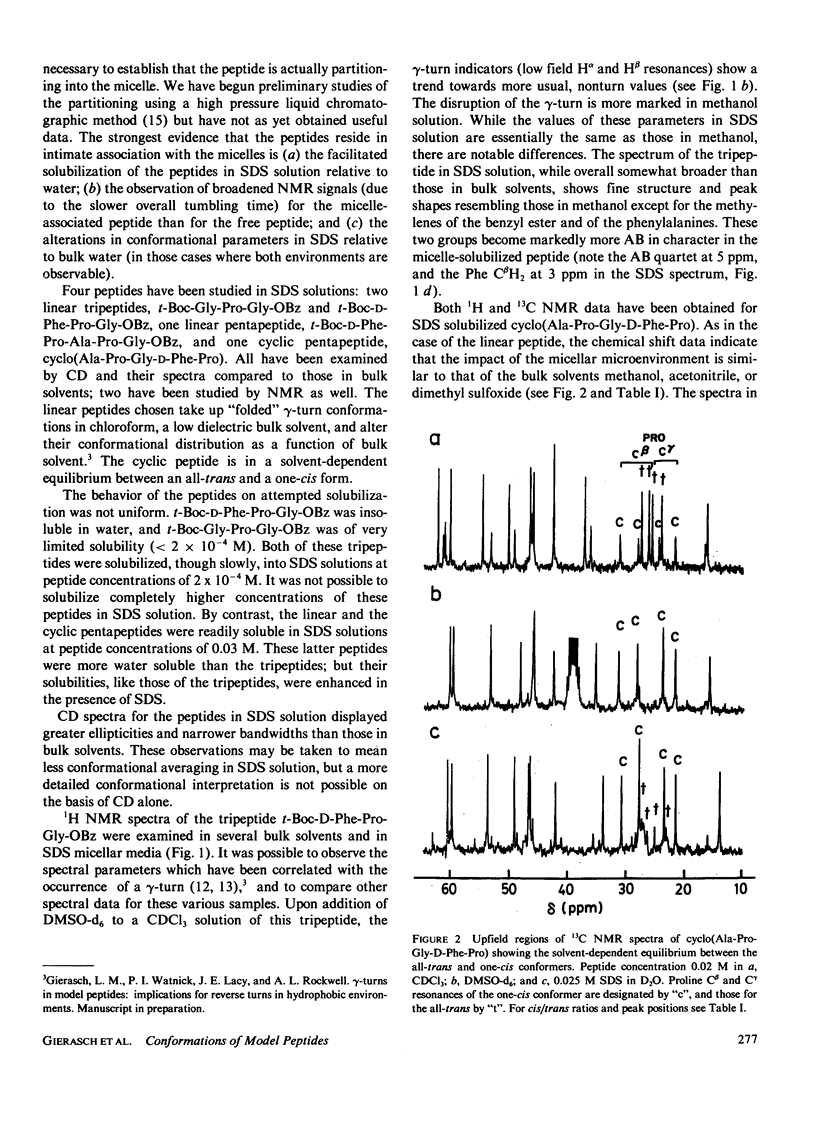
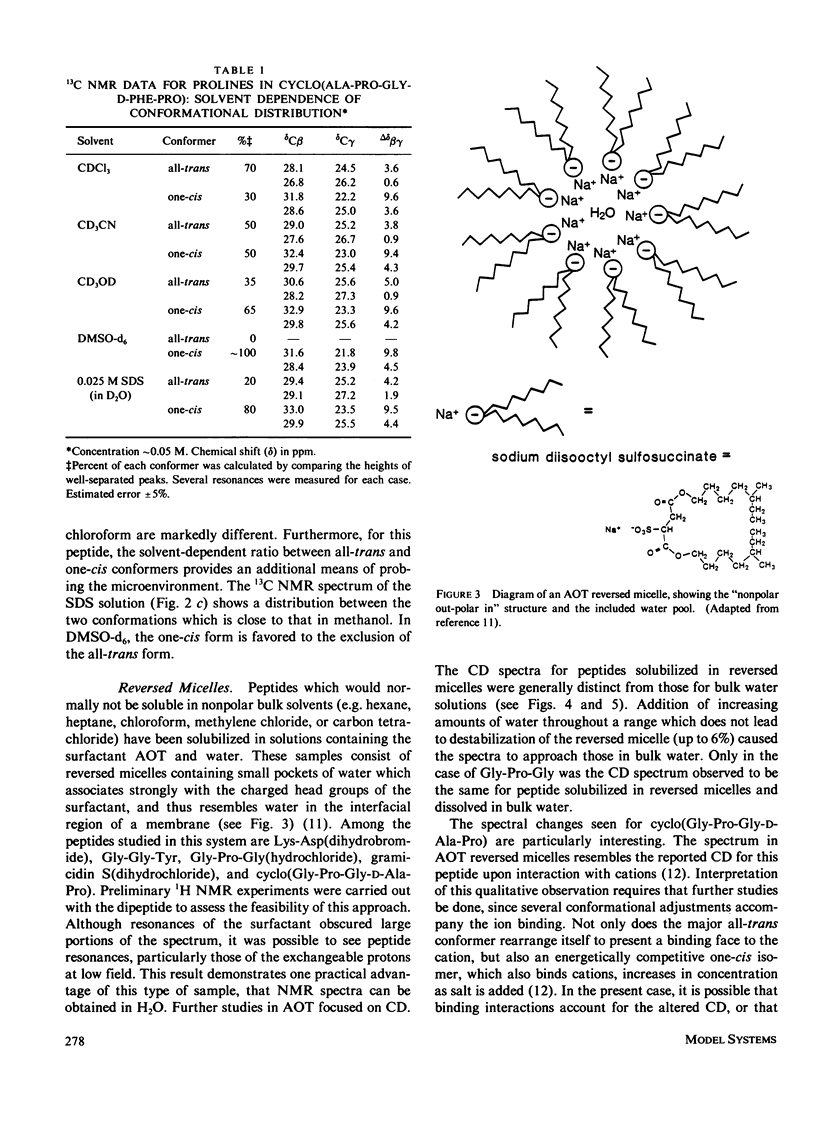
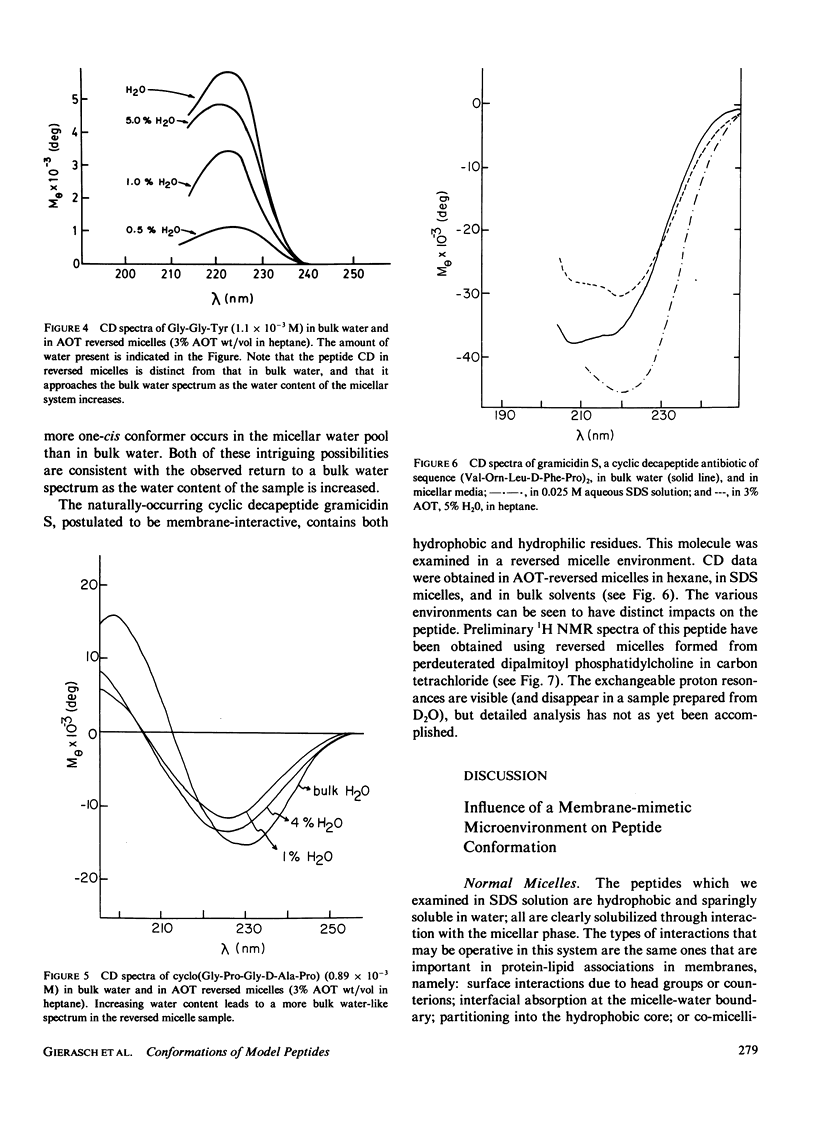
The influence of a membrane environment on the conformational energetics of a polypeptide chain has been investigated through studies of model peptides in a variety of membrane-mimetic media. Nuclear magnetic resonance (NMR) and circular dichroism (CD) data have been obtained for the peptides in bulk hydrophobic solvents, normal micelles, and reversed micelles. Several hydrophobic peptides which are sparingly soluble in water have been solubilized in aqueous sodium dodecyl sulfate (SDS) solution. NMR and CD data indicate that the micelle-solubilized peptides experience an environment with the conformational impact of bulk methanol, and have decreased conformational freedom. The site of residence of the peptides interacting with the micelles appears to be near the surfactant head groups, in a region permeated by water, and not in the micelle core. Strongly hydrophilic peptides have been solubilized in nonpolar solvents by reversed micelles. These peptides are located in small water pools in close association with the head groups of the surfactant. NMR and CD data show that there is a conformational impact of this interfacial water region on peptide solubilizates distinct from that of bulk water.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cullis P. R., de Kruijff B. Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim Biophys Acta. 1979 Dec 20;559(4):399–420. doi: 10.1016/0304-4157(79)90012-1. [DOI] [PubMed] [Google Scholar]
- Feigenson G. W., Meers P. R. 1H NMR study of valinomycin conformation in a phospholipid bilayer. Nature. 1980 Jan 17;283(5744):313–314. doi: 10.1038/283313a0. [DOI] [PubMed] [Google Scholar]
- Hagen D. S., Weiner J. H., Sykes B. D. Investigation of solvent accessibility of the fluorotyrosyl residues of M13 coat protein in deoxycholate micelles and phospholipid vesicles. Biochemistry. 1979 May 15;18(10):2007–2012. doi: 10.1021/bi00577a026. [DOI] [PubMed] [Google Scholar]
- Henrikson K. P. Observations by nuclear magnetic resonance of the interactions of water with lecithin micelles in carbon tetrachloride solution. Biochim Biophys Acta. 1970 Apr 21;203(2):228–232. doi: 10.1016/0005-2736(70)90136-7. [DOI] [PubMed] [Google Scholar]
- KAUZMANN W. Some factors in the interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63. doi: 10.1016/s0065-3233(08)60608-7. [DOI] [PubMed] [Google Scholar]
- Knöppel E., Eisenberg D., Wickner W. Interactions of melittin, a preprotein model, with detergents. Biochemistry. 1979 Sep 18;18(19):4177–4181. doi: 10.1021/bi00586a021. [DOI] [PubMed] [Google Scholar]
- Kuntz I. D. Protein folding. J Am Chem Soc. 1972 May 31;94(11):4009–4012. doi: 10.1021/ja00766a060. [DOI] [PubMed] [Google Scholar]
- Lauterwein J., Bösch C., Brown L. R., Wüthrich K. Physicochemical studies of the protein-lipid interactions in melittin-containing micelles. Biochim Biophys Acta. 1979 Sep 21;556(2):244–264. doi: 10.1016/0005-2736(79)90046-4. [DOI] [PubMed] [Google Scholar]
- SINGLETON W. S., GRAY M. S., BROWN M. L., WHITE J. L. CHROMATOGRAPHICALLY HOMOGENEOUS LECITHIN FROM EGG PHOSPHOLIPIDS. J Am Oil Chem Soc. 1965 Jan;42:53–56. doi: 10.1007/BF02558256. [DOI] [PubMed] [Google Scholar]
- Wallace B. A., Blout E. R. Conformation of an oligopeptide in phospholipid vesicles. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1775–1779. doi: 10.1073/pnas.76.4.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein S., Wallace B. A., Morrow J. S., Veatch W. R. Conformation of the gramicidin A transmembrane channel: A 13C nuclear magnetic resonance study of 13C-enriched gramicidin in phosphatidylcholine vesicles. J Mol Biol. 1980 Oct 15;143(1):1–19. doi: 10.1016/0022-2836(80)90121-7. [DOI] [PubMed] [Google Scholar]
- Wu C. S., Lee N. M., Loh H. H., Yang J. T., Li C. H. beta-Endorphin: formation of alpha-helix in lipid solutions. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3656–3659. doi: 10.1073/pnas.76.8.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]


