Abstract
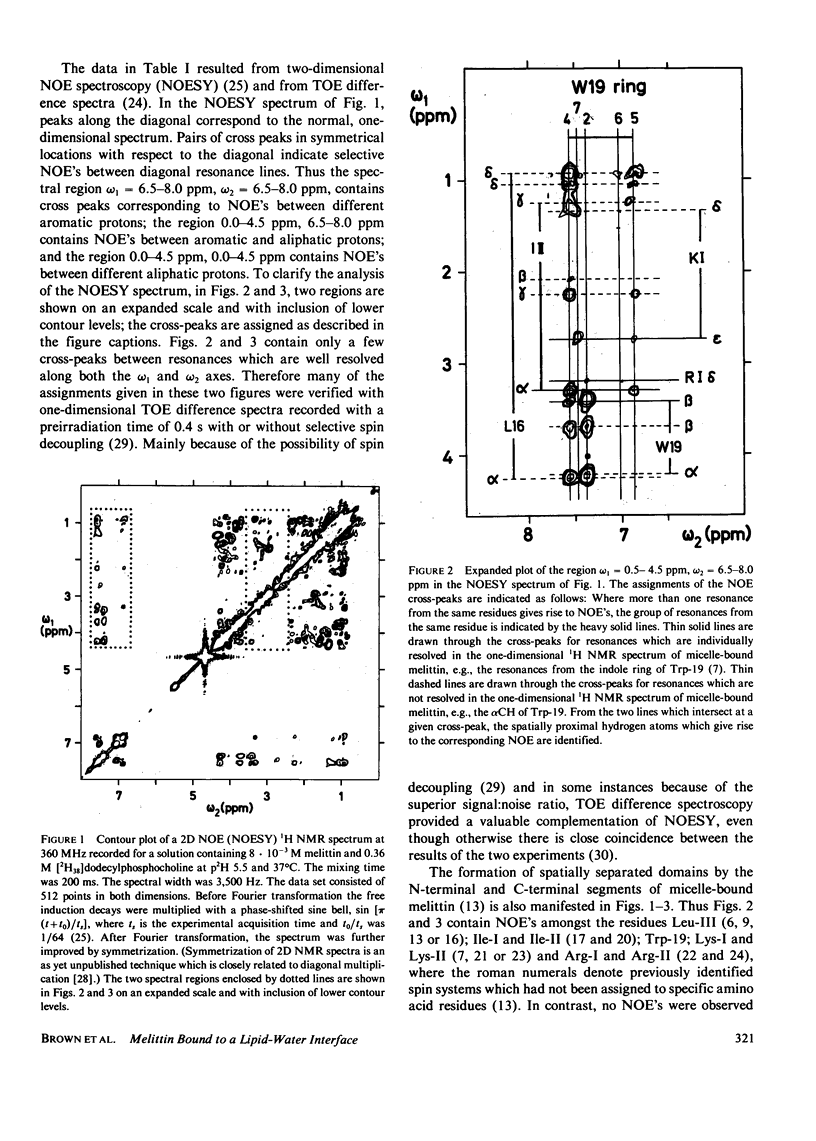
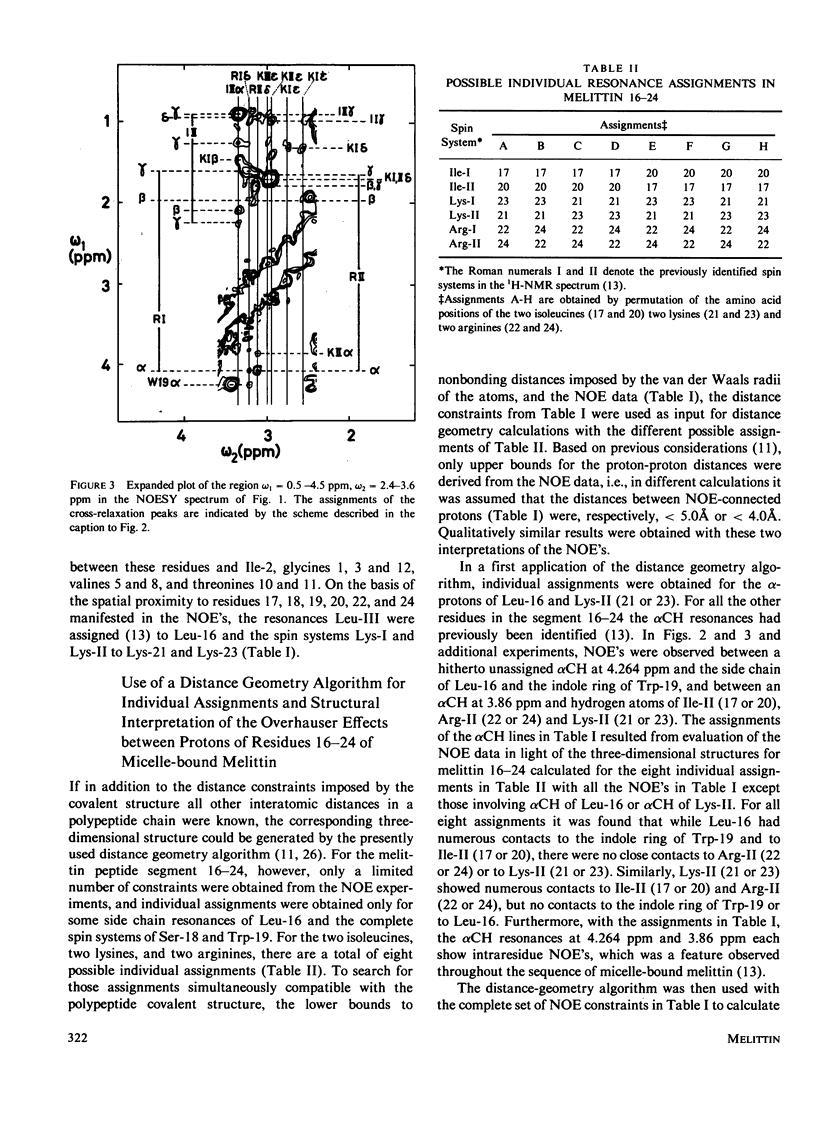
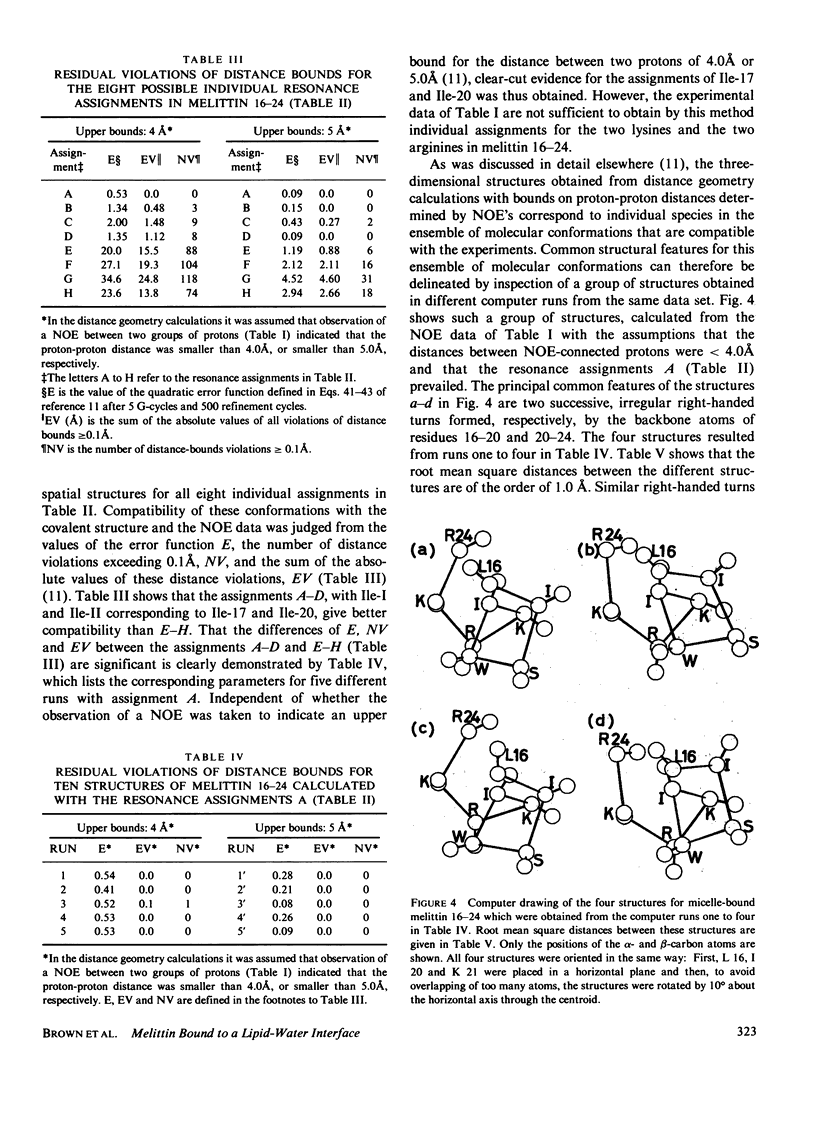
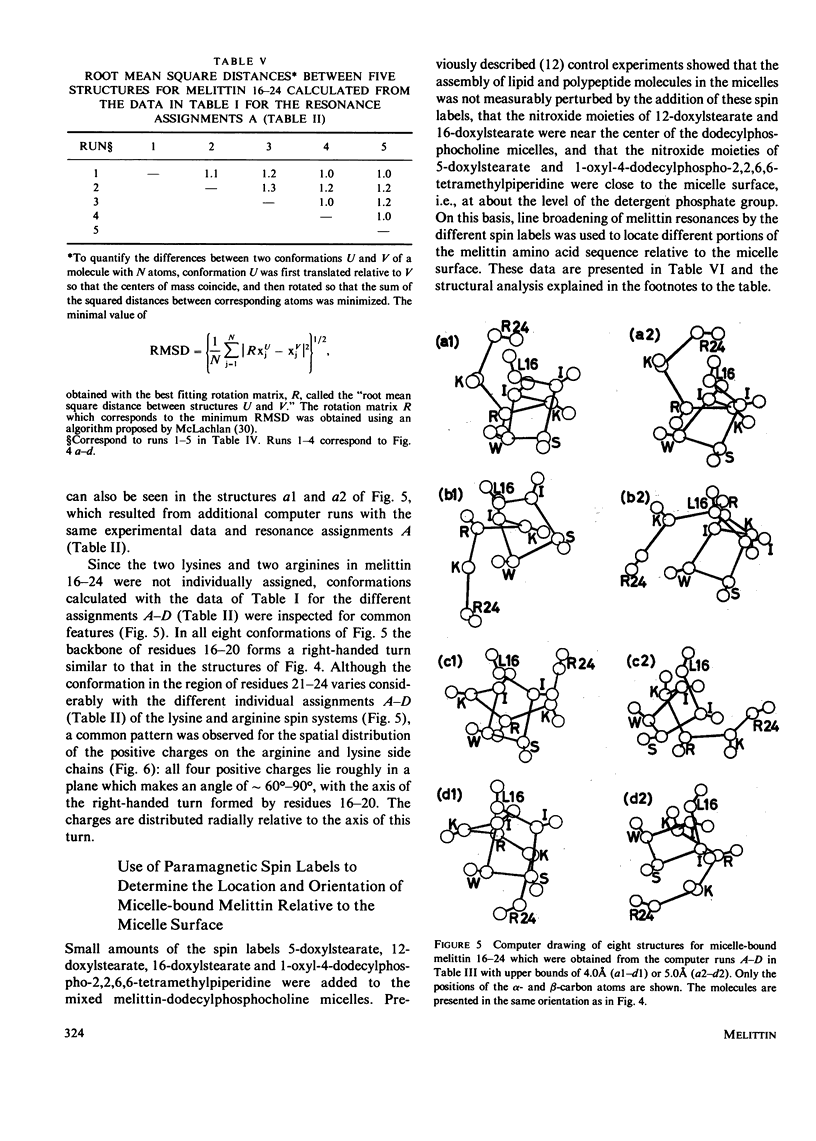
Previously, the size and stoichiometry of mixed micelles of perdeuterated dodecylphosphocholine and melittin were characterized and the 1H NMR spin systems of most amino acid residues of micelle-bound melittin identified. One- and two-dimensional 1H-1H Overhauser experiments have now been used to obtain qualitative information on intramolecular proton-proton distances. These data show that the N-terminal and the C-terminal segments of melittin form two spatially distinct, compact domains; using lipid spin labels these could be located near the micelle surface. For the C-terminal domain a detailed conformation was determined by using the distance contraints from the Overhauser studies as input for a distance geometry algorithm.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun W., Bösch C., Brown L. R., Go N., Wüthrich K. Combined use of proton-proton Overhauser enhancements and a distance geometry algorithm for determination of polypeptide conformations. Application to micelle-bound glucagon. Biochim Biophys Acta. 1981 Feb 27;667(2):377–396. doi: 10.1016/0005-2795(81)90205-1. [DOI] [PubMed] [Google Scholar]
- Brown L. R., Bösch C., Wüthrich K. Location and orientation relative to the micelle surface for glucagon in mixed micelles with dodecylphosphocholine: EPR and NMR studies. Biochim Biophys Acta. 1981 Apr 6;642(2):296–312. doi: 10.1016/0005-2736(81)90447-8. [DOI] [PubMed] [Google Scholar]
- Brown L. R., Lauterwein J., Wüthrich K. High-resolution 1H-NMR studies of self-aggregation of melittin in aqueous solution. Biochim Biophys Acta. 1980 Apr 25;622(2):231–244. doi: 10.1016/0005-2795(80)90034-3. [DOI] [PubMed] [Google Scholar]
- Brown L. R. Use of fully deuterated micelles for conformational studies of membrane proteins by high resolution 1H nuclear magnetic resonance. Biochim Biophys Acta. 1979 Oct 19;557(1):135–148. doi: 10.1016/0005-2736(79)90096-8. [DOI] [PubMed] [Google Scholar]
- Brown L. R., Wüthrich K. Melittin bound to dodecylphosphocholine micelles. H-NMR assignments and global conformational features. Biochim Biophys Acta. 1981 Sep 21;647(1):95–111. doi: 10.1016/0005-2736(81)90298-4. [DOI] [PubMed] [Google Scholar]
- Bösch C., Brown L. R., Wüthrich K. Physicochemical characterization of glucagon-containing lipid micelles. Biochim Biophys Acta. 1980 Dec 12;603(2):298–312. doi: 10.1016/0005-2736(80)90376-4. [DOI] [PubMed] [Google Scholar]
- Chapman D., Gómez-Fernández J. C., Goñi F. M. Intrinsic protein--lipid interactions. Physical and biochemical evidence. FEBS Lett. 1979 Feb 15;98(2):211–223. doi: 10.1016/0014-5793(79)80186-6. [DOI] [PubMed] [Google Scholar]
- Cherry R. J. Rotational and lateral diffusion of membrane proteins. Biochim Biophys Acta. 1979 Dec 20;559(4):289–327. doi: 10.1016/0304-4157(79)90009-1. [DOI] [PubMed] [Google Scholar]
- Dawson C. R., Drake A. F., Helliwell J., Hider R. C. The interaction of bee melittin with lipid bilayer membranes. Biochim Biophys Acta. 1978 Jun 16;510(1):75–86. doi: 10.1016/0005-2736(78)90131-1. [DOI] [PubMed] [Google Scholar]
- Habermann E. Bee and wasp venoms. Science. 1972 Jul 28;177(4046):314–322. doi: 10.1126/science.177.4046.314. [DOI] [PubMed] [Google Scholar]
- Lad P. L., Shier W. T. Effect of melittin-induced membrane alterations on rat heart adenylate cyclase activity. Arch Biochem Biophys. 1980 Oct 15;204(2):418–424. doi: 10.1016/0003-9861(80)90052-1. [DOI] [PubMed] [Google Scholar]
- Lauterwein J., Brown L. R., Wüthrich K. High-resolution 1H-NMR studies of monomeric melittin in aqueous solution. Biochim Biophys Acta. 1980 Apr 25;622(2):219–230. doi: 10.1016/0005-2795(80)90033-1. [DOI] [PubMed] [Google Scholar]
- Lauterwein J., Bösch C., Brown L. R., Wüthrich K. Physicochemical studies of the protein-lipid interactions in melittin-containing micelles. Biochim Biophys Acta. 1979 Sep 21;556(2):244–264. doi: 10.1016/0005-2736(79)90046-4. [DOI] [PubMed] [Google Scholar]
- Lavialle F., Levin I. W., Mollay C. Interaction of melittin with dimyristoyl phosphatidylcholine liposomes: evidence for boundary lipid by Raman spectroscopy. Biochim Biophys Acta. 1980 Jul 16;600(1):62–71. doi: 10.1016/0005-2736(80)90411-3. [DOI] [PubMed] [Google Scholar]
- McLachlan A. D. Gene duplications in the structural evolution of chymotrypsin. J Mol Biol. 1979 Feb 15;128(1):49–79. doi: 10.1016/0022-2836(79)90308-5. [DOI] [PubMed] [Google Scholar]
- Mollay C., Kreil G., Berger H. Action of phospholipases on the cytoplasmic membrane of Escherichia coli. Stimulation by melittin. Biochim Biophys Acta. 1976 Mar 5;426(2):317–324. doi: 10.1016/0005-2736(76)90340-0. [DOI] [PubMed] [Google Scholar]
- Nagayama K., Wüthrich K., Ernst R. R. Two-dimensional spin echo correlated spectroscopy (SECSY) for 1H NMR studies of biological macromolecules. Biochem Biophys Res Commun. 1979 Sep 12;90(1):305–311. doi: 10.1016/0006-291x(79)91625-5. [DOI] [PubMed] [Google Scholar]
- Olson F. C., Munjal D., Malviya A. N. Structural and respiratory effects of melittin (Apis mellifera) on rat liver mitochondria. Toxicon. 1974 Aug;12(4):419–425. doi: 10.1016/0041-0101(74)90010-5. [DOI] [PubMed] [Google Scholar]
- Seelig J., Seelig A. Lipid conformation in model membranes and biological membranes. Q Rev Biophys. 1980 Feb;13(1):19–61. doi: 10.1017/s0033583500000305. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Henkart P. Fluidity of cell membranes--current concepts and trends. Int Rev Cytol. 1979;60:121–147. [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Wickner W. The assembly of proteins into biological membranes: The membrane trigger hypothesis. Annu Rev Biochem. 1979;48:23–45. doi: 10.1146/annurev.bi.48.070179.000323. [DOI] [PubMed] [Google Scholar]
- Williams J. C., Bell R. M. Membrane matrix disruption by melittin. Biochim Biophys Acta. 1972 Nov 2;288(2):255–262. doi: 10.1016/0005-2736(72)90246-5. [DOI] [PubMed] [Google Scholar]
- Wüthrich K., Bösch C., Brown L. R. Conformational studies of lipid-bound polypeptides by elucidation of proton-proton cross-relaxation networks. Biochem Biophys Res Commun. 1980 Aug 29;95(4):1504–1509. doi: 10.1016/s0006-291x(80)80067-2. [DOI] [PubMed] [Google Scholar]
- Yunes R., Goldhammer A. R., Garner W. K., Cordes E. H. Phospholipases: melittin facilitation of bee venom phospholipase A2-catalyzed hydrolysis of unsonicated lecithin liposomes. Arch Biochem Biophys. 1977 Sep;183(1):105–112. doi: 10.1016/0003-9861(77)90424-6. [DOI] [PubMed] [Google Scholar]
- de Bony J., Dufourcq J., Clin B. Lipid-protein interactions: NMR study of melittin and its binding to lysophosphatidylcholine. Biochim Biophys Acta. 1979 Apr 19;552(3):531–534. doi: 10.1016/0005-2736(79)90197-4. [DOI] [PubMed] [Google Scholar]


