Abstract
The cytotoxic peptide from honeybee venom, melittin, and a synthetic peptide analogue of it lyse human erythrocytes in a biphasic process. The kinetics of the lysis in 0.30 M sucrose, 0.01 M sodium phosphate, pH 7.30 at 4 degrees C were investigated. Our results show that melittin rapidly binds to the outer surface of the erythrocyte membrane, and the surface-bound monomers produce transient openings through which approximately 40 hemoglobin molecules can escape. Concomitantly, the melittin loses its ability to effect the process, presumably by translocation through the bilayer. The half-life for this process is 1.2 min. In a much slower process, dimers of this internalized melittin again produce transient membrane openings in a steady state. On a molar basis, the synthetic peptide analogue produces a fast process comparable to that caused by melittin, but is more efficient in the slow phase. Escape of hemoglobin and of carbonic anhydrase through the openings is diffusion controlled. These results suggest that the functional units necessary for the activity of melittin-like cytotoxic peptides are a 20 amino acid amphiphilic alpha-helix with a hydrophobic:hydrophilic ratio greater than 1 and a short segment with a high concentration of positive charges.
Full text
PDF
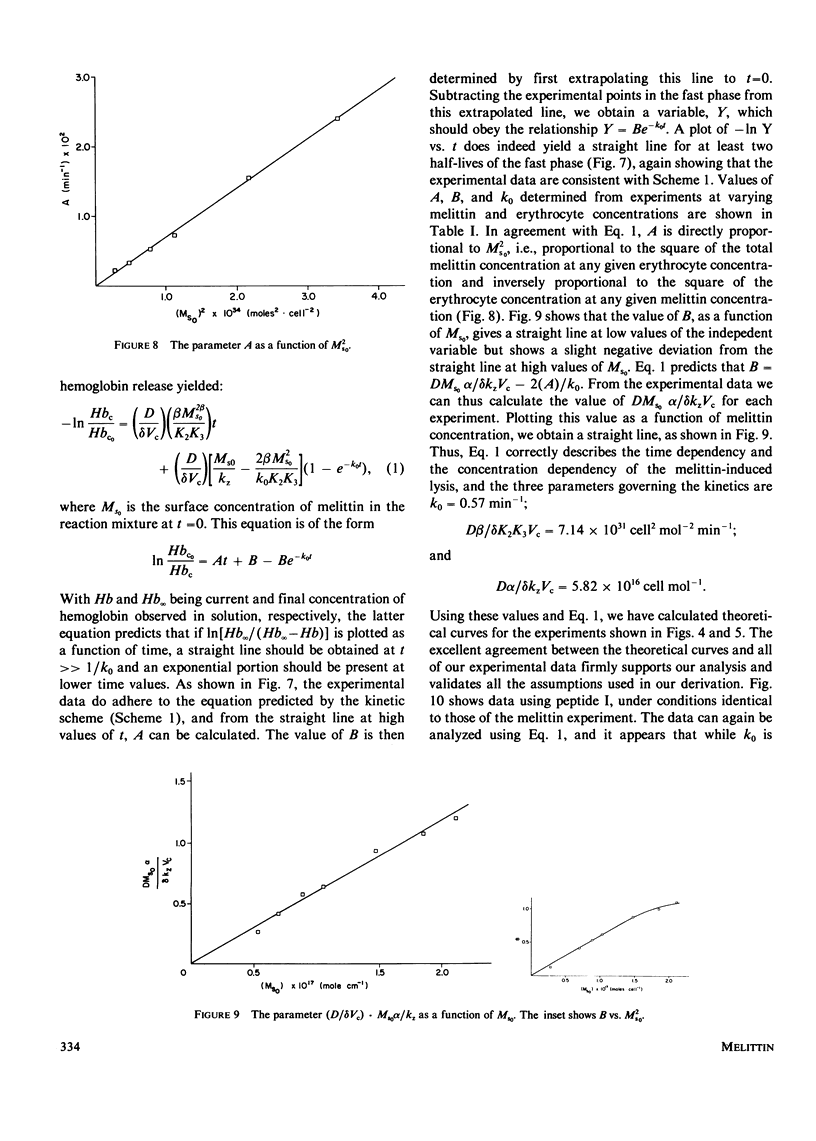
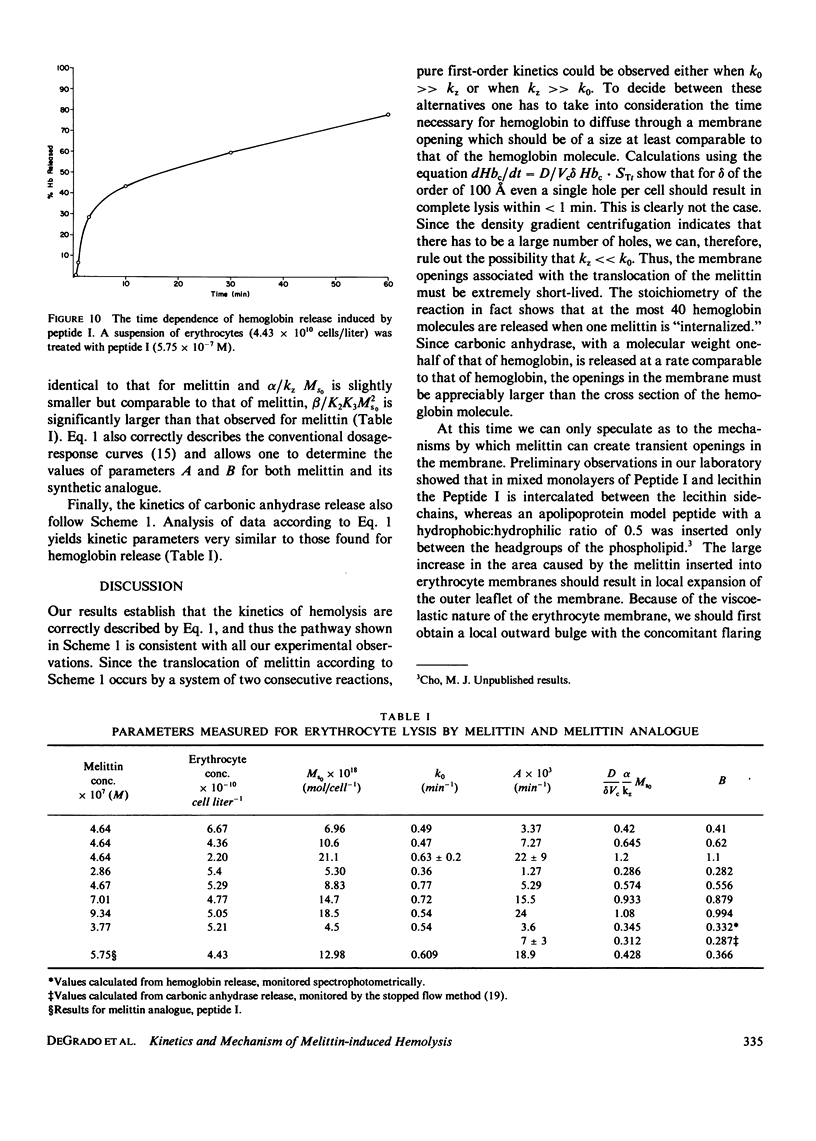

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dalmark M., Wieth J. O. Temperature dependence of chloride, bromide, iodide, thiocyanate and salicylate transport in human red cells. J Physiol. 1972 Aug;224(3):583–610. doi: 10.1113/jphysiol.1972.sp009914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson C. R., Drake A. F., Helliwell J., Hider R. C. The interaction of bee melittin with lipid bilayer membranes. Biochim Biophys Acta. 1978 Jun 16;510(1):75–86. doi: 10.1016/0005-2736(78)90131-1. [DOI] [PubMed] [Google Scholar]
- Drake A. F., Hider R. C. The structure of melittin in lipid bilayer membranes. Biochim Biophys Acta. 1979 Aug 7;555(2):371–373. doi: 10.1016/0005-2736(79)90178-0. [DOI] [PubMed] [Google Scholar]
- Edelstein C., Kézdy F. J., Scanu A. M., Shen B. W. Apolipoproteins and the structural organization of plasma lipoproteins: human plasma high density lipoprotein-3. J Lipid Res. 1979 Feb;20(2):143–153. [PubMed] [Google Scholar]
- Epand R. M., Jones A. J., Schreier S. Interaction of glucagon with dimyristoyl glycerophosphocholine. Biochim Biophys Acta. 1977 Mar 28;491(1):296–304. doi: 10.1016/0005-2795(77)90065-4. [DOI] [PubMed] [Google Scholar]
- Fitch W. M. Phylogenies constrained by the crossover process as illustrated by human hemoglobins and a thirteen-cycle, eleven-amino-acid repeat in human apolipoprotein A-I. Genetics. 1977 Jul;86(3):623–644. doi: 10.1093/genetics/86.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn R. B., Dalmark M., Tosteson D. C., Wieth J. O. Characteristics of chloride transport in human red blood cells. J Gen Physiol. 1973 Feb;61(2):185–206. doi: 10.1085/jgp.61.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann E., Kowallek H. Modifikationen der Aminogruppen und des Tryptophans im Melittin als Mittel zur Erkennung von Struktur-Wirkungs-Beziehungen. Hoppe Seylers Z Physiol Chem. 1970 Jul;351(7):884–890. [PubMed] [Google Scholar]
- Hall R. L., Vennesland B., Kézdy F. J. Glyoxylate carboligase of Escherichia coli. Identification of carbon dioxide as the primary reaction product. J Biol Chem. 1969 Aug 10;244(15):3991–3998. [PubMed] [Google Scholar]
- Knöppel E., Eisenberg D., Wickner W. Interactions of melittin, a preprotein model, with detergents. Biochemistry. 1979 Sep 18;18(19):4177–4181. doi: 10.1021/bi00586a021. [DOI] [PubMed] [Google Scholar]
- Osborne J. C., Jr, Brewer H. B., Jr The plasma lipoproteins. Adv Protein Chem. 1977;31:253–337. doi: 10.1016/s0065-3233(08)60220-x. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Jackson R. L., Morrisett J. D., Gotto A. M., Jr A molecular theory of lipid-protein interactions in the plasma lipoproteins. FEBS Lett. 1974 Jan 15;38(3):247–258. doi: 10.1016/0014-5793(74)80064-5. [DOI] [PubMed] [Google Scholar]
- Shen B. W., Scanu A. M., Kézdy F. J. Structure of human serum lipoproteins inferred from compositional analysis. Proc Natl Acad Sci U S A. 1977 Mar;74(3):837–841. doi: 10.1073/pnas.74.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. C., Pownall H. J., Gotto A. M., Jr The plasma lipoproteins: structure and metabolism. Annu Rev Biochem. 1978;47:751–757. doi: 10.1146/annurev.bi.47.070178.003535. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Kant J. A. Preparation of impermeable ghosts and inside-out vesicles from human erythrocyte membranes. Methods Enzymol. 1974;31:172–180. doi: 10.1016/0076-6879(74)31019-1. [DOI] [PubMed] [Google Scholar]
- Yokoyama S., Fukushima D., Kupferberg J. P., Kézdy F. J., Kaiser E. T. The mechanism of activation of lecithin:cholesterol acyltransferase by apolipoprotein A-I and an amphiphilic peptide. J Biol Chem. 1980 Aug 10;255(15):7333–7339. [PubMed] [Google Scholar]
- Yokoyama S., Fukushima D., Kupferberg J. P., Kézdy F. J., Kaiser E. T. The mechanism of activation of lecithin:cholesterol acyltransferase by apolipoprotein A-I and an amphiphilic peptide. J Biol Chem. 1980 Aug 10;255(15):7333–7339. [PubMed] [Google Scholar]



