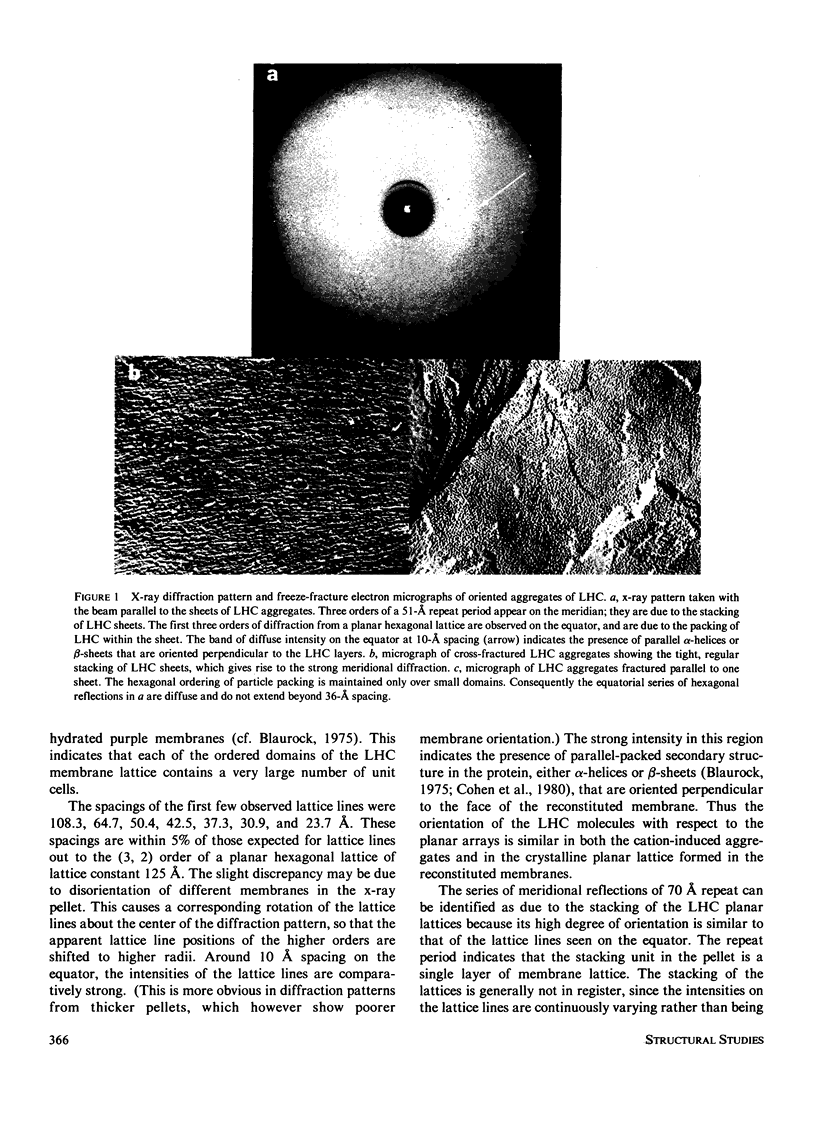
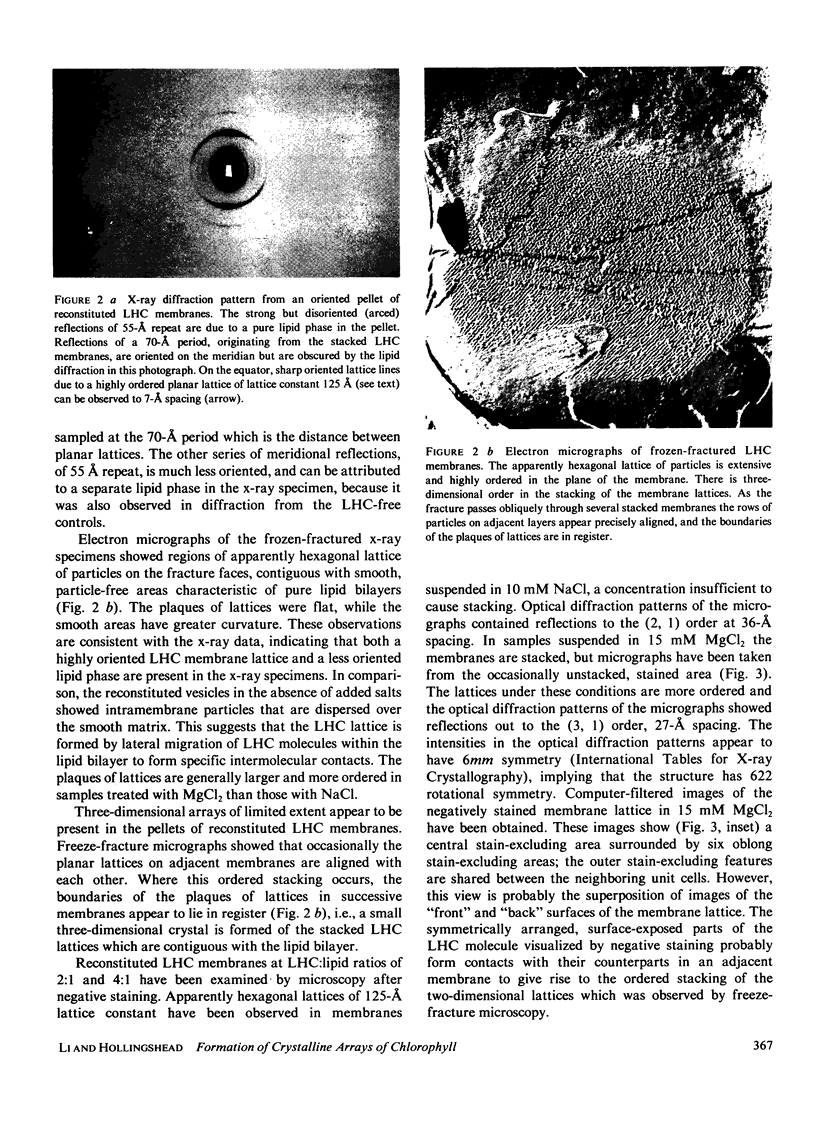
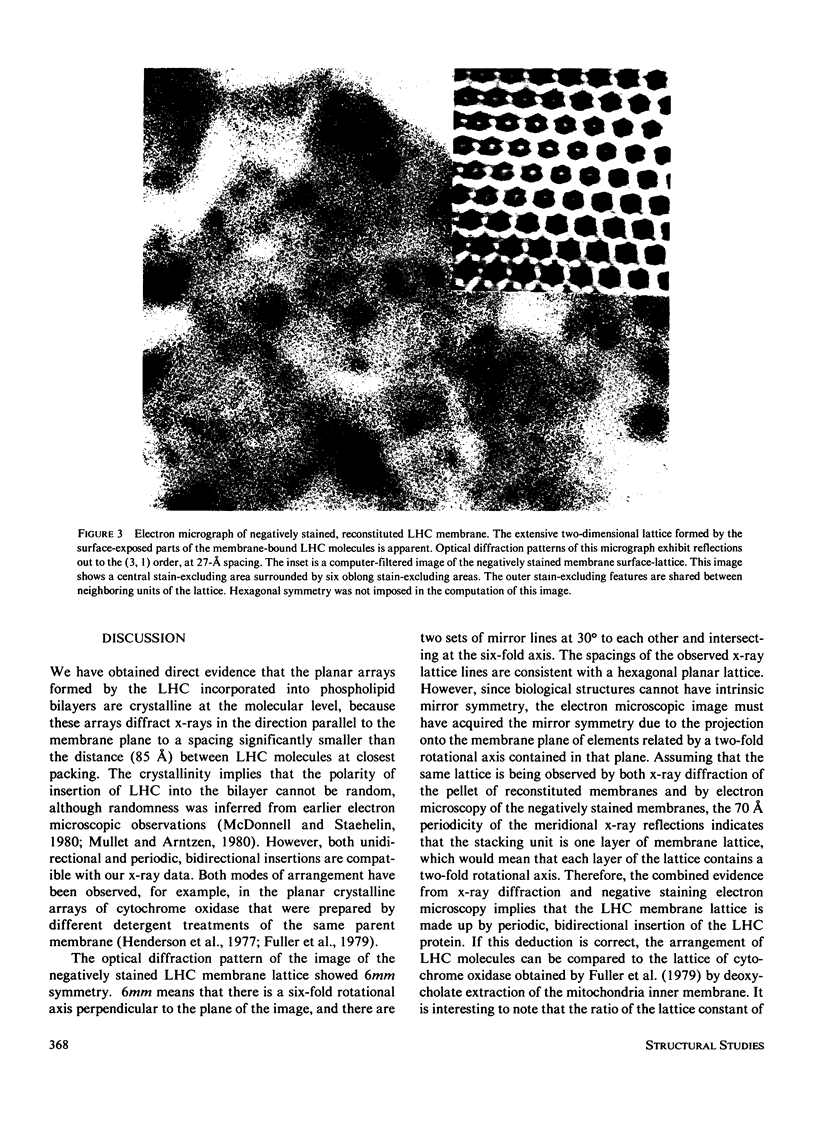
Abstract
The structure of the major protein constituent of photosynthetic membranes in higher plants, the chlorophyll a/b-light harvesting complex (LHC), was studied by x-ray diffraction and electron microscopy. The LHC was purified from Triton X-100 solubilized thylakoid membranes of the pea, and contained 6 mol of chlorophylls a and b per mole of a polypeptide of 27,000 molecular weight. X-ray diffraction showed that in the presence of 10 mM MgCl2, purified LHC forms planar aggregates that stack with a period of 51 A. Within each layer, LHC molecules pack with a center-to-center distance of 85 A but without long-range order. However, when LHC is incorporated into single-walled vesicles of plant lecithin, the addition of NaCl above 10 mM, or MgCl2 above 2 mM, led to the formation of plaques of hexagonal lattices, with a lattice constant of 125 A. The large domain size and high degree of order in the plane of the membrane are evident from the sharp lattice lines observed to 7 A resolution on the equator of the x-ray pattern. Freeze-fracture electron micrographs demonstrated an aligned stacking of the lattices in adjacent membranes, resulting in crystallinity in the third dimension over short distances. Micrographs of negatively stained membranes revealed a hexagonal lattice of the same lattice constant, formed by surface-exposed parts of the LHC molecules which are probably responsible for the ordered stacking of lattices. In both the LHC aggregates and in the reconstituted membrane lattices the diffracted x-ray intensities at 10-A spacing on the equator indicate that the LHC molecule contains paralled alpha-helices or beta-sheets that are oriented perpendicular to the planar arrays.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M., Levine R. P. The relationship between chlorophyll-protein complexes and chloroplast membrane polypeptides. Biochim Biophys Acta. 1974 Jul 25;357(1):118–126. doi: 10.1016/0005-2728(74)90117-0. [DOI] [PubMed] [Google Scholar]
- Anderson J. M. The molecular organization of chloroplast thylakoids. Biochim Biophys Acta. 1975 Aug 15;416(2):191–235. doi: 10.1016/0304-4173(75)90007-5. [DOI] [PubMed] [Google Scholar]
- Apel K. Chlorophyll-proteins from Acetabularia mediterranea. Brookhaven Symp Biol. 1976 Jun 7;(28):149–161. [PubMed] [Google Scholar]
- Armond P. A., Arntzen C. J., Briantais J. M., Vernotte C. Differentiation of chloroplast lamellae. Light harvesting efficiency and grana development. Arch Biochem Biophys. 1976 Jul;175(1):54–63. doi: 10.1016/0003-9861(76)90484-7. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arntzen C. J., Armond P. A., Briantais J. M., Burke J. J., Novitzky W. P. Dynamic interactions among structural components of the chloroplast membrane. Brookhaven Symp Biol. 1976 Jun 7;(28):316–337. [PubMed] [Google Scholar]
- Berg S., Dodge S., Krogmann D. W., Dilley R. A. Chloroplast grana membrane carboxyl groups: their involvement in membrane association. Plant Physiol. 1974 Apr;53(4):619–627. doi: 10.1104/pp.53.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaurock A. E. Bacteriorhodospin: a trans-membrane pump containing alpha-helix. J Mol Biol. 1975 Apr 5;93(2):139–158. doi: 10.1016/0022-2836(75)90124-2. [DOI] [PubMed] [Google Scholar]
- Burke J. J., Ditto C. L., Arntzen C. J. Involvement of the light-harvesting complex in cation regulation of excitation energy distribution in chloroplasts. Arch Biochem Biophys. 1978 Apr 15;187(1):252–263. doi: 10.1016/0003-9861(78)90031-0. [DOI] [PubMed] [Google Scholar]
- Chua N. H., Blobel G., Siekevitz P., Palade G. E. Periodic variations in the ratio of free to thylakoid-bound chloroplast ribosomes during the cell cycle of Chlamydomonas reinhardtii. J Cell Biol. 1976 Nov;71(2):497–514. doi: 10.1083/jcb.71.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen F. E., Sternberg M. J., Taylor W. R. Analysis and prediction of protein beta-sheet structures by a combinatorial approach. Nature. 1980 Jun 5;285(5764):378–382. doi: 10.1038/285378a0. [DOI] [PubMed] [Google Scholar]
- Fisher R. G., Woods N. E., Fuchs H. E., Sweet R. M. Three-dimensional structures of C-phycocyanin and B-phycoerythrin at 5-A resolution. J Biol Chem. 1980 Jun 10;255(11):5082–5089. [PubMed] [Google Scholar]
- Fuller S. D., Capaldi R. A., Henderson R. Structure of cytochrome c oxidase in deoxycholate-drived two-dimensional crystals. J Mol Biol. 1979 Oct 25;134(2):305–327. doi: 10.1016/0022-2836(79)90037-8. [DOI] [PubMed] [Google Scholar]
- Gerritsen W. J., Verkley A. J., Zwaal R. F., Van Deenen L. L. Freeze-fracture appearance and disposition of band 3 protein from the human erythrocyte membrane in lipid vesicles. Eur J Biochem. 1978 Apr;85(1):255–261. doi: 10.1111/j.1432-1033.1978.tb12234.x. [DOI] [PubMed] [Google Scholar]
- Giddings T. H., Withers N. W., Staehelin L. A. Supramolecular structure of stacked and unstacked regions of the photosynthetic membranes of Prochloron sp., a prokaryote. Proc Natl Acad Sci U S A. 1980 Jan;77(1):352–356. doi: 10.1073/pnas.77.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R., Capaldi R. A., Leigh J. S. Arrangement of cytochrome oxidase molecules in two-dimensional vesicle crystals. J Mol Biol. 1977 Jun 5;112(4):631–648. doi: 10.1016/s0022-2836(77)80167-8. [DOI] [PubMed] [Google Scholar]
- Holloway P. W. A simple procedure for removal of Triton X-100 from protein samples. Anal Biochem. 1973 May;53(1):304–308. doi: 10.1016/0003-2697(73)90436-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matthews B. W., Fenna R. E., Bolognesi M. C., Schmid M. F., Olson J. M. Structure of a bacteriochlorophyll a-protein from the green photosynthetic bacterium Prosthecochloris aestuarii. J Mol Biol. 1979 Jun 25;131(2):259–285. doi: 10.1016/0022-2836(79)90076-7. [DOI] [PubMed] [Google Scholar]
- McDonnel A., Staehelin L. A. Adhesion between liposomes mediated by the chlorophyll a/b light-harvesting complex isolated from chloroplast membranes. J Cell Biol. 1980 Jan;84(1):40–56. doi: 10.1083/jcb.84.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. R., Miller G. J., McIntyre K. R. The light-harvesting chlorpohyll-protein complex of photosystem II. Its location in the photosynthetic membrane. J Cell Biol. 1976 Nov;71(2):624–638. doi: 10.1083/jcb.71.2.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullet J. E., Arntzen C. J. Simulation of grana stacking in a model membrane system. Mediation by a purified light-harvesting pigment-protein complex from chloroplasts. Biochim Biophys Acta. 1980 Jan 4;589(1):100–117. doi: 10.1016/0005-2728(80)90135-8. [DOI] [PubMed] [Google Scholar]
- Ojakian G. K., Satir P. Particle movements in chloroplast membranes: quantitative measurements of membrane fluidity by the freeze-fracture technique. Proc Natl Acad Sci U S A. 1974 May;71(5):2052–2056. doi: 10.1073/pnas.71.5.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin L. A., Armond P. A., Miller K. R. Chloroplast membrane organization at the supramolecular level and its functional implications. Brookhaven Symp Biol. 1976 Jun 7;(28):278–315. [PubMed] [Google Scholar]
- Staehelin L. A. Reversible particle movements associated with unstacking and restacking of chloroplast membranes in vitro. J Cell Biol. 1976 Oct;71(1):136–158. doi: 10.1083/jcb.71.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornber J. P., Stewart J. C., Hatton M. W., Bailey J. L. Studies on the nature of chloroplast lamellae. II. Chemical composition and further physical properties of two chlorophyll-protein complexes. Biochemistry. 1967 Jul;6(7):2006–2014. doi: 10.1021/bi00859a019. [DOI] [PubMed] [Google Scholar]
- Unwin P. N., Henderson R. Molecular structure determination by electron microscopy of unstained crystalline specimens. J Mol Biol. 1975 May 25;94(3):425–440. doi: 10.1016/0022-2836(75)90212-0. [DOI] [PubMed] [Google Scholar]