Abstract
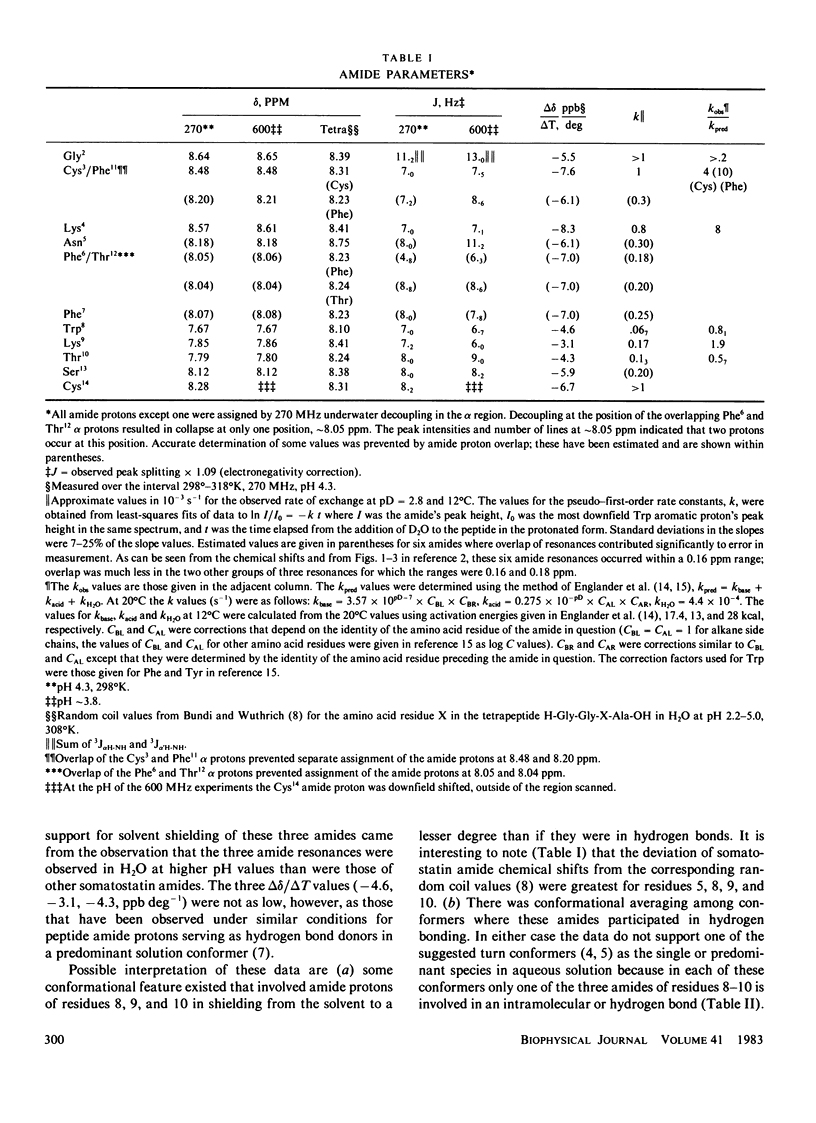
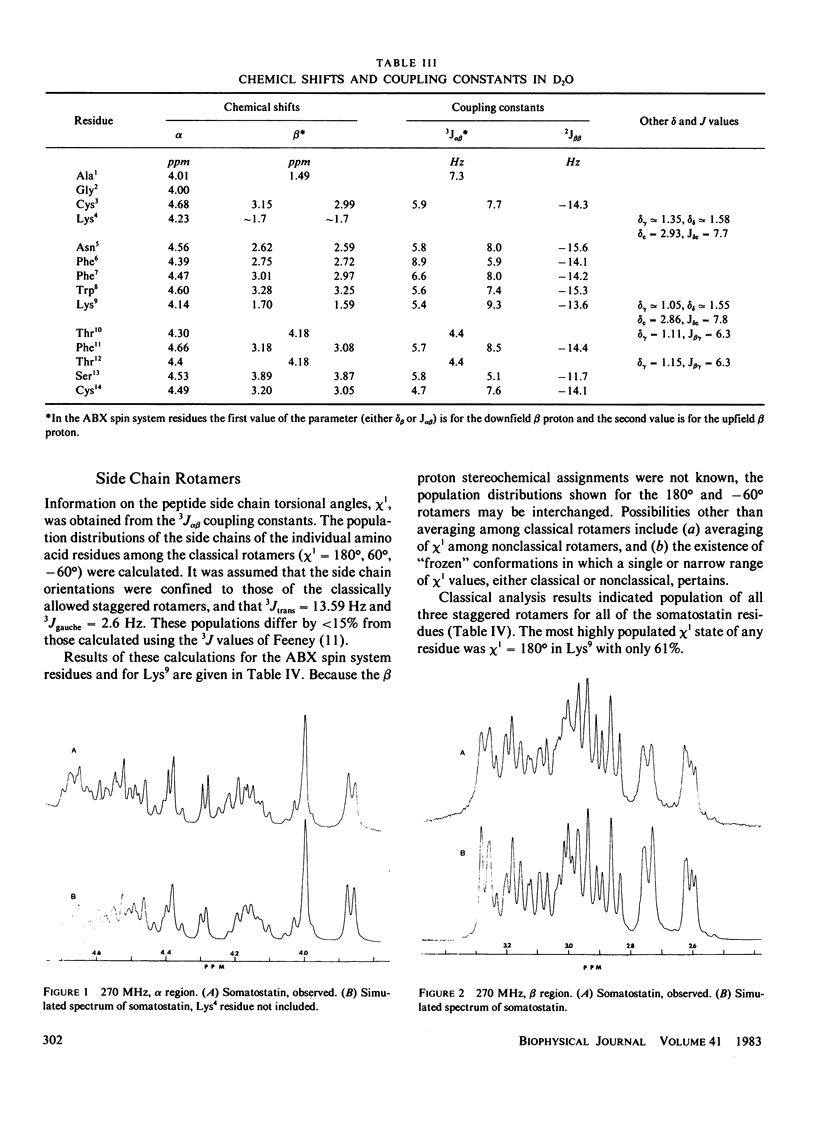
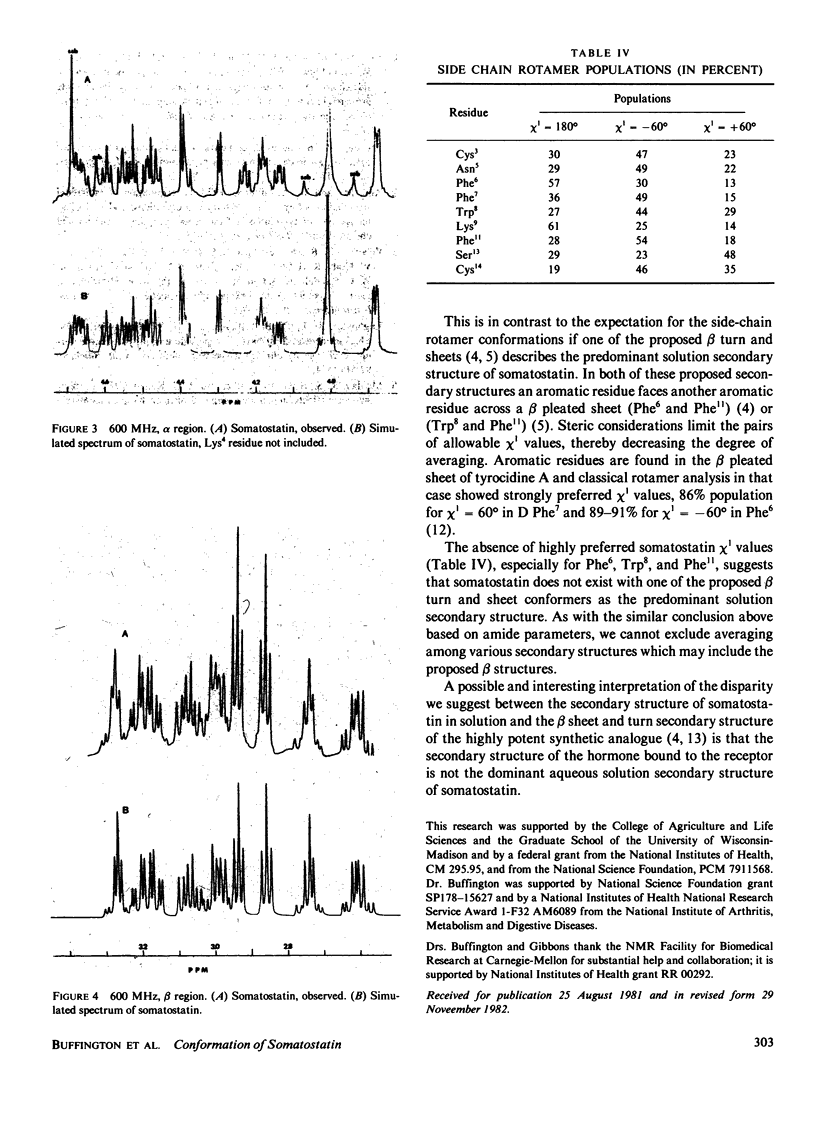
The conformation of the 14 amino acid peptide hormone somatostatin in aqueous solution was investigated through a proton magnetic resonance (PMR) scalar coupling analysis. Experiments were performed at two fields, 270 and 600 MHz, and included double and triple resonance difference scalar decoupling, resolution enhancement and computer simulation. The agreement between simulated and observed spectra at both fields provided support for the correctness of the analysis. The resultant scalar coupling constants, 3J alpha H-NH and 3J alpha B, gave information on the backbone (phi) and side chain (chi 1) torsional angles, respectively, which eliminated either of the proposed conformations of somatostatin as describing a predominant conformer of the molecule in solution under our conditions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buffington L., Garsky V., Massiot G., Rivier J., Gibbons W. A. Assignment of the 270 MHz nuclear magnetic resonance spectrum of somatostatin in water. Biochem Biophys Res Commun. 1980 Mar 28;93(2):376–384. doi: 10.1016/0006-291x(80)91087-6. [DOI] [PubMed] [Google Scholar]
- Campbell I. D., Dobson C. M. The application of high resolution nuclear magnetic resonance to biological systems. Methods Biochem Anal. 1979;25:1–133. doi: 10.1002/9780470110454.ch1. [DOI] [PubMed] [Google Scholar]
- Crawford J. L., Lipscomb W. N., Schellman C. G. The reverse turn as a polypeptide conformation in globular proteins. Proc Natl Acad Sci U S A. 1973 Feb;70(2):538–542. doi: 10.1073/pnas.70.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englander J. J., Calhoun D. B., Englander S. W. Measurement and calibration of peptide group hydrogen-deuterium exchange by ultraviolet spectrophotometry. Anal Biochem. 1979 Jan 15;92(2):517–524. doi: 10.1016/0003-2697(79)90693-6. [DOI] [PubMed] [Google Scholar]
- Hallenga K., van Binst G., Scarso A., Michel A., Knappenberg M., Dremier C., Brison J., Dirkx J. The conformational properties of the peptide hormone somatostatin (III). Assignment and analysis of the 1H and 13C high resolution NMR spectra of somatostatin in aqueous solution. FEBS Lett. 1980 Sep 22;119(1):47–52. doi: 10.1016/0014-5793(80)80995-1. [DOI] [PubMed] [Google Scholar]
- Holladay L. A., Rivier J., Puett D. Conformational studies on somatostatin and analogues. Biochemistry. 1977 Nov 1;16(22):4895–4900. doi: 10.1021/bi00641a024. [DOI] [PubMed] [Google Scholar]
- Kuo M. C., Gibbons W. A. Determination of individual side-chain conformations, tertiary conformations, and molecular topography of tyrocidine A from scalar coupling constants and chemical shifts. Biochemistry. 1979 Dec 25;18(26):5855–5867. doi: 10.1021/bi00593a016. [DOI] [PubMed] [Google Scholar]
- Kuo M., Jones C. R., Mahn T. H., Miller P. R., Nicholls L. J., Gibbons W. A. Simplification and spin-spin analysis of the side chain proton magnetic resonance spectrum of the decapeptide gramicidin S using difference scalar decoupling and biosynthesis of specifically deuterated analogs. J Biol Chem. 1979 Oct 25;254(20):10301–10306. [PubMed] [Google Scholar]
- Molday R. S., Englander S. W., Kallen R. G. Primary structure effects on peptide group hydrogen exchange. Biochemistry. 1972 Jan 18;11(2):150–158. doi: 10.1021/bi00752a003. [DOI] [PubMed] [Google Scholar]
- Redfield A. G. Proton nuclear magnetic resonance in aqueous solutions. Methods Enzymol. 1978;49:253–270. doi: 10.1016/s0076-6879(78)49014-7. [DOI] [PubMed] [Google Scholar]
- Veber D. F., Freidlinger R. M., Perlow D. S., Paleveda W. J., Jr, Holly F. W., Strachan R. G., Nutt R. F., Arison B. H., Homnick C., Randall W. C. A potent cyclic hexapeptide analogue of somatostatin. Nature. 1981 Jul 2;292(5818):55–58. doi: 10.1038/292055a0. [DOI] [PubMed] [Google Scholar]


