Abstract
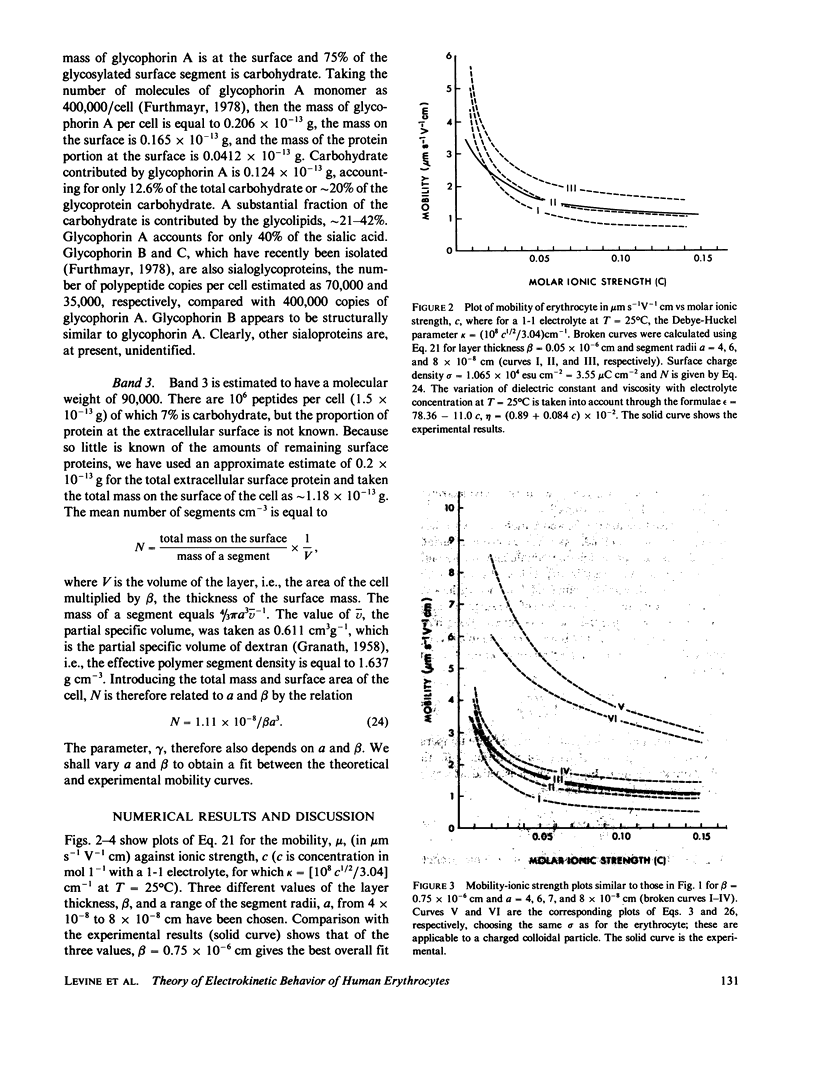
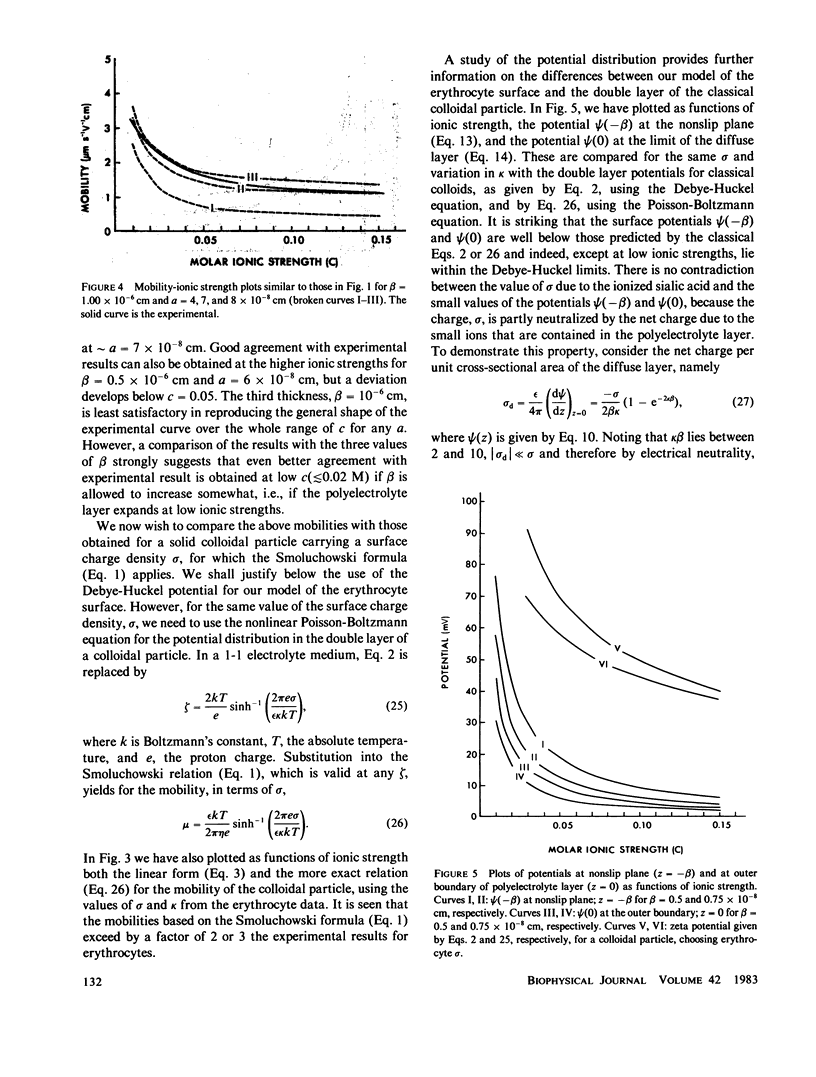
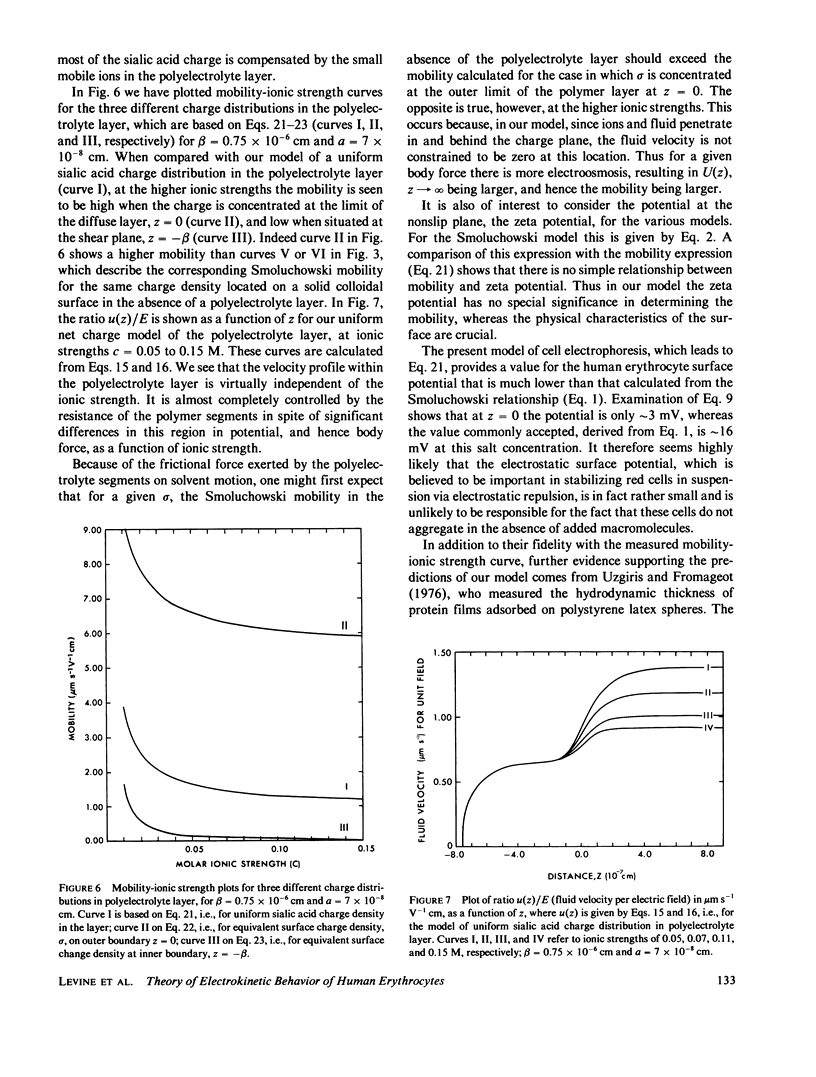
We develop a theory of electrophoresis of human erythrocytes that predicts mobilities significantly smaller than those based on the classical Smoluchowski relation. In the classical treatment the charge is assumed to be spread uniformly on the hydrodynamic surface. The present model takes into account that most of the charge, due mainly to sialic acid, is contained in the glycocalyx. The glycocalyx is modeled as a permeable layer of polyelectrolyte molecules anchored to the cell membrane. The charge is assumed to be uniformly distributed throughout this layer. The fluid flow in the layer is treated as being dominated by Stokes friction arising from idealized polymer segments. The Navier-Stokes equations are solved to give the dependence of electroosomotic velocity with distance from the cell surface. An expression for the electrophoretic mobility is obtained which contains two parameters (a) the thickness of the glycocalyx and (b) the mean polymer segment radius. The best fit to experimental data is obtained if these are given the values 75 A and 7 A, respectively. Deviation from experimental data at low ionic strength (less than 0.05 M) occurs. However, this deviation is in the direction one would expect if at low ionic strength the polyelectrolyte layer expands slightly due to decreased charge shielding.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- EYLAR E. H., MADOFF M. A., BRODY O. V., ONCLEY J. L. The contribution of sialic acid to the surface charge of the erythrocyte. J Biol Chem. 1962 Jun;237:1992–2000. [PubMed] [Google Scholar]
- Furthmayr H. Glycophorins A, B, and C: a family of sialoglycoproteins. Isolation and preliminary characterization of trypsin derived peptides. J Supramol Struct. 1978;9(1):79–95. doi: 10.1002/jss.400090109. [DOI] [PubMed] [Google Scholar]
- HAYDON D. A. The surface charge of cells and some other small particles as indicated by electrophoresis. II. The interpretation of the electrophoretic charge. Biochim Biophys Acta. 1961 Jul 8;50:457–462. doi: 10.1016/0006-3002(61)90004-x. [DOI] [PubMed] [Google Scholar]
- HEARD D. H., SEAMAN G. V. The influence of pH and ionic strength on the electrokinetic stability of the human erythrocyte membrane. J Gen Physiol. 1960 Jan;43:635–654. doi: 10.1085/jgp.43.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILLIER J., HOFFMAN J. F. On the ultrastructure of the plasma membrane as determined by the electron microscope. J Cell Physiol. 1953 Oct;42(2):203–247. doi: 10.1002/jcp.1030420205. [DOI] [PubMed] [Google Scholar]
- Jenkins R. E., Tanner M. J. Ionic-strength-dependent changes in the structure of the major protein of the human erythrocyte membrane. Biochem J. 1977 Jan 1;161(1):131–138. doi: 10.1042/bj1610131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järnefelt J., Rush J., Li Y. T., Laine R. A. Erythroglycan, a high molecular weight glycopeptide with the repeating structure [galactosyl-(1 leads to 4)-2-deoxy-2-acetamido-glucosyl(1 leads to 3)] comprising more than one-third of the protein-bound carbohydrate of human erythrocyte stroma. J Biol Chem. 1978 Nov 25;253(22):8006–8009. [PubMed] [Google Scholar]
- Luner S. J., Sturgeon P., Szklarek D., McQuiston D. T. Effects of proteases and neuraminidase on RBC surface charge and agglutination. A kinetic study. Vox Sang. 1975;28(3):184–199. doi: 10.1111/j.1423-0410.1975.tb02757.x. [DOI] [PubMed] [Google Scholar]
- Seaman G. V., Knox R. J., Nordt F. J., Regan D. H. Red cell aging. I. Surface charge density and sialic acid content of density-fractionated human erythrocytes. Blood. 1977 Dec;50(6):1001–1011. [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita M., Marchesi V. T. Amino-acid sequence and oligosaccharide attachment sites of human erythrocyte glycophorin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2964–2968. doi: 10.1073/pnas.72.8.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzgiris E. E., Fromageot H. P. Thickness and density of protein films by optical mixing spectroscopy. Biopolymers. 1976 Feb;15(2):257–263. doi: 10.1002/bip.1976.360150204. [DOI] [PubMed] [Google Scholar]
- Vassar P. S., Hards J. M., Seaman G. V. Surface properties of human lymphocytes. Biochim Biophys Acta. 1973 Jan 2;291(1):107–115. doi: 10.1016/0005-2736(73)90065-5. [DOI] [PubMed] [Google Scholar]
- WESTERMAN M. P., PIERCE L. E., JENSEN W. N. A direct method for the quantitative measurement of red cell dimensions. J Lab Clin Med. 1961 May;57:819–824. [PubMed] [Google Scholar]


