Abstract
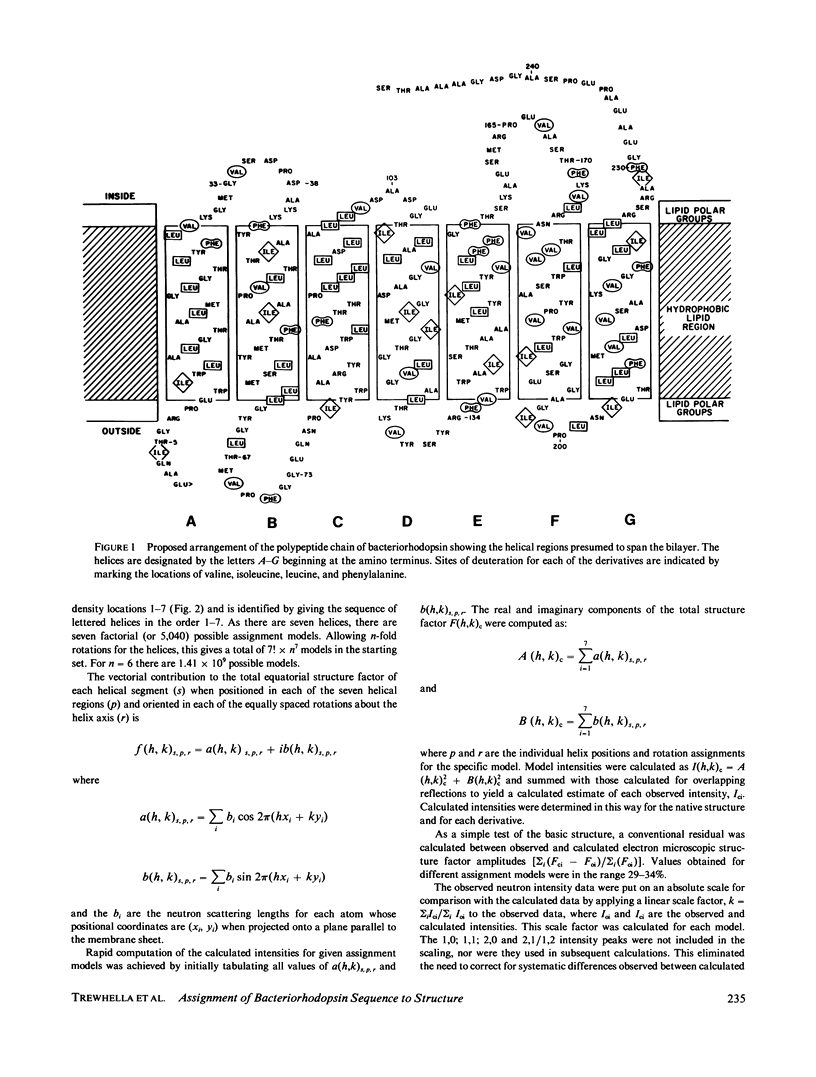
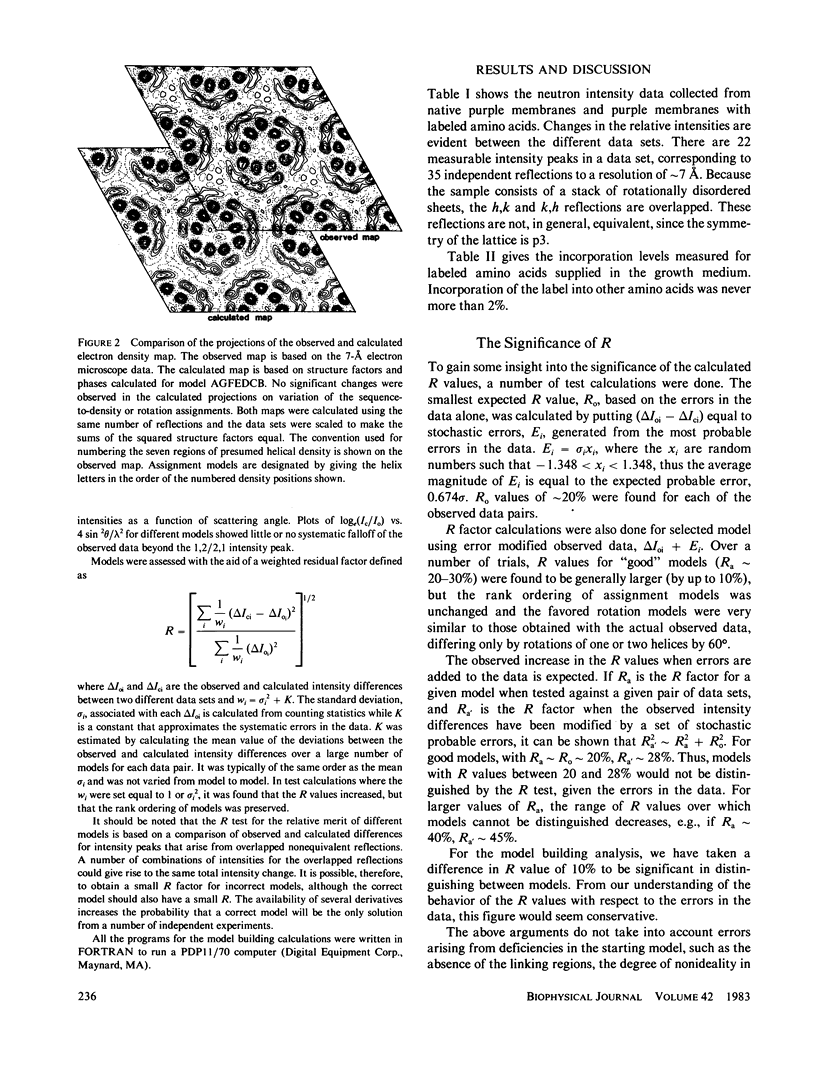
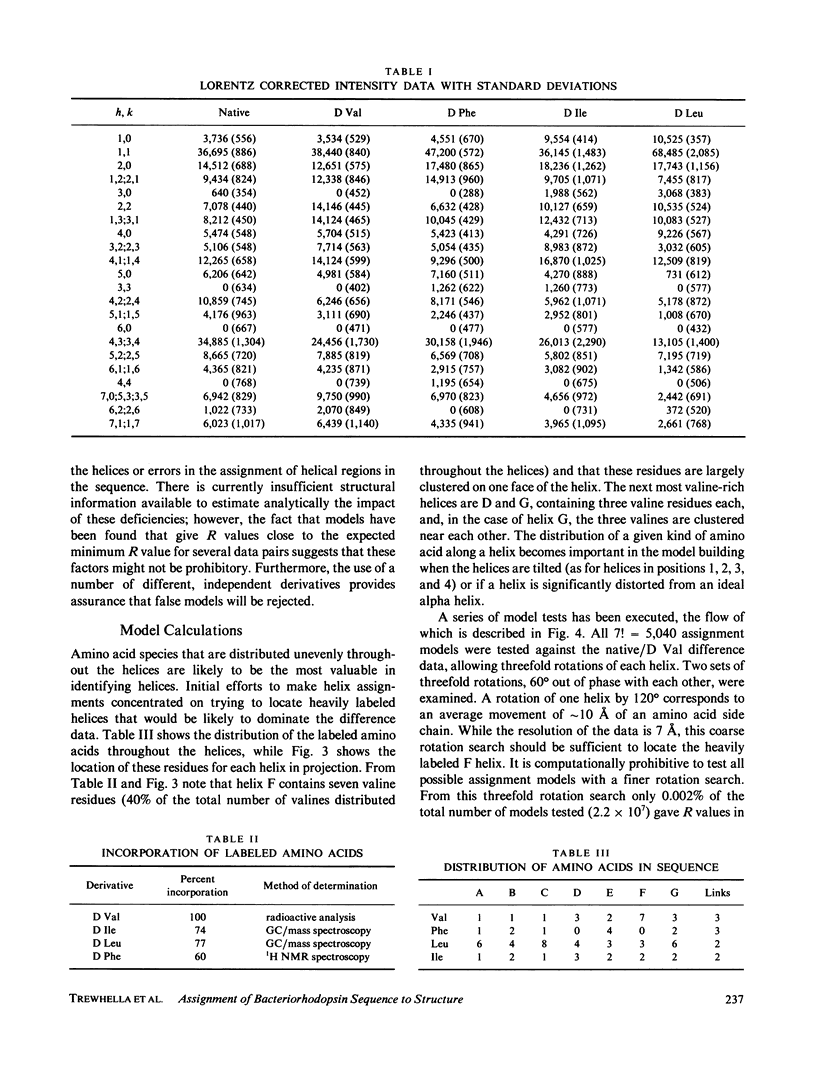
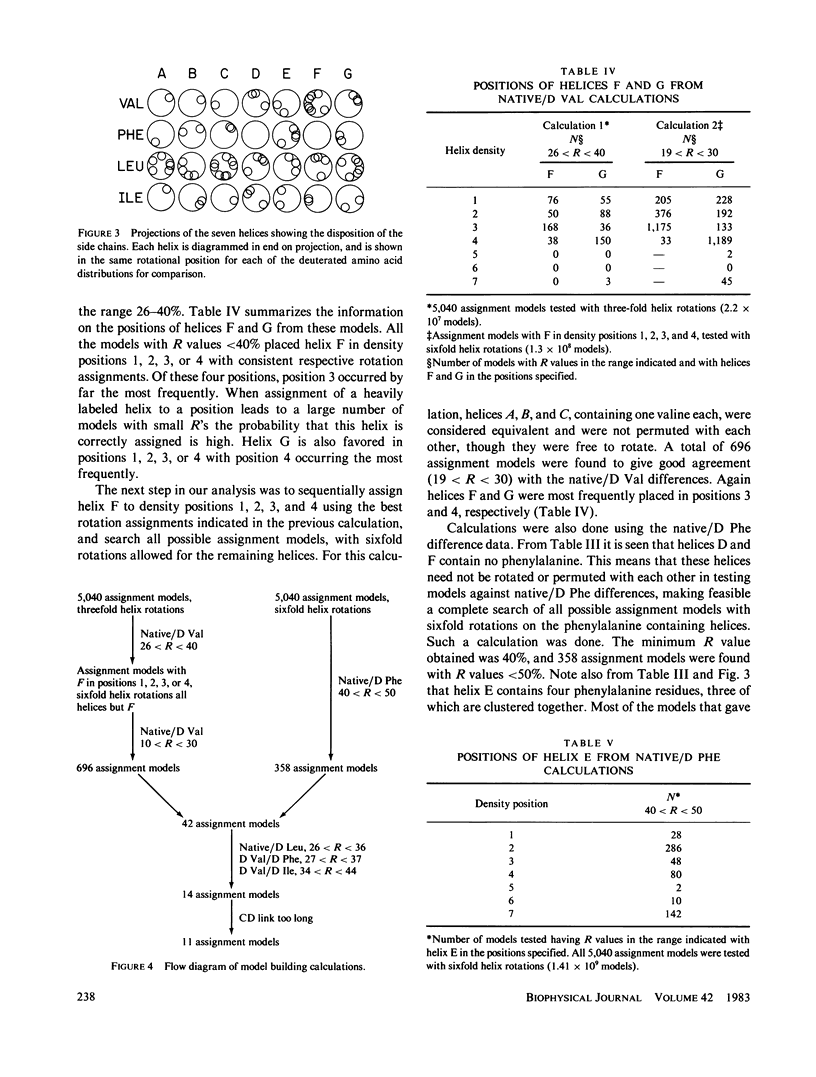
Specific amino acid sequence segments have been assigned to locations in the structural map of bacteriorhodopsin using two-dimensional neutron diffraction data and a model building analysis. Models are constructed computationally by building specific regions of the amino acid sequence as alpha helices and then positioning the helices on axes indicated by the density map of Henderson and Unwin (Nature [Lond.]. 1975, 257:28-32). Neutron diffraction data were collected from samples of stacked, oriented "native" purple membranes as well as purple membranes containing different kinds of deuterated amino acids. Models differing in the assignments of helices to specific axes and in rotations of the helices about those axes were tested against the neutron data using a weighted residual factor to rank the models. This residual factor was calculated between observed and predicted intensity differences for pairs of data sets. Using this approach, a small set of related models has been found that predicts the observed intensity changes between five independent data sets. These models are inconsistent with the proposed locations of the retinal chromophore and the carboxyl terminus and with any of the previously proposed models for bacteriorhodopsin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agard D. A., Stroud R. M. Linking regions between helices in bacteriorhodopsin revealed. Biophys J. 1982 Mar;37(3):589–602. [PMC free article] [PubMed] [Google Scholar]
- Bayley H., Huang K. S., Radhakrishnan R., Ross A. H., Takagaki Y., Khorana H. G. Site of attachment of retinal in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2225–2229. doi: 10.1073/pnas.78.4.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman D. M., Henderson R., McLachlan A. D., Wallace B. A. Path of the polypeptide in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2023–2027. doi: 10.1073/pnas.77.4.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman D. M., Zaccai G. Bacteriorhodopsin is an inside-out protein. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5894–5898. doi: 10.1073/pnas.77.10.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehreke C. W., Nakamoto H., Zumwalt R. W. Gas-liquid chromatography of protein amino acid trimethylsilyl derivatives. J Chromatogr. 1969 Nov 25;45(1):24–51. doi: 10.1016/s0021-9673(01)86179-3. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Keefer L. M., Bradshaw R. A. Structural studies on Halobacterium halobium bacteriorhodopsin. Fed Proc. 1977 May;36(6):1799–1804. [PubMed] [Google Scholar]
- Khorana H. G., Gerber G. E., Herlihy W. C., Gray C. P., Anderegg R. J., Nihei K., Biemann K. Amino acid sequence of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5046–5050. doi: 10.1073/pnas.76.10.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. I., Stoekenius W., Crespi H. L., Schoenborn B. P. The location of retinal in the purple membrane profile by neutron diffraction. J Mol Biol. 1979 Jun 5;130(4):395–404. doi: 10.1016/0022-2836(79)90430-3. [DOI] [PubMed] [Google Scholar]
- LeMaster D. M., Richards F. M. Preparative-scale isolation of isotopically labeled amino acids. Anal Biochem. 1982 May 15;122(2):238–247. doi: 10.1016/0003-2697(82)90275-5. [DOI] [PubMed] [Google Scholar]
- Lemke H. D., Oesterhelt D. Lysine 216 is a binding site of the retinyl moiety in bacteriorhodopsin. FEBS Lett. 1981 Jun 15;128(2):255–260. doi: 10.1016/0014-5793(81)80093-2. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971 Sep 29;233(39):149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov Y. A., Abdulaev N. G., Feigina M. Y., Kiselev A. V., Lobanov N. A. The structural basis of the functioning of bacteriorhodopsin: an overview. FEBS Lett. 1979 Apr 15;100(2):219–224. doi: 10.1016/0014-5793(79)80338-5. [DOI] [PubMed] [Google Scholar]
- Rogan P. K., Zaccai G. Hydration in purple membrane as a function of relative humidity. J Mol Biol. 1981 Jan 5;145(1):281–284. doi: 10.1016/0022-2836(81)90344-2. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Lozier R. H., Bogomolni R. A. Bacteriorhodopsin and the purple membrane of halobacteria. Biochim Biophys Acta. 1979 Mar 14;505(3-4):215–278. doi: 10.1016/0304-4173(79)90006-5. [DOI] [PubMed] [Google Scholar]
- Wallace B. A., Henderson R. Location of the carboxyl terminus of bacteriorhodopsin in purple membrane. Biophys J. 1982 Sep;39(3):233–239. doi: 10.1016/S0006-3495(82)84513-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccai G., Gilmore D. J. Areas of hydration in the purple membrane of Halobacterium halobium: a neutron diffraction study. J Mol Biol. 1979 Aug 5;132(2):181–191. doi: 10.1016/0022-2836(79)90390-5. [DOI] [PubMed] [Google Scholar]



