Abstract
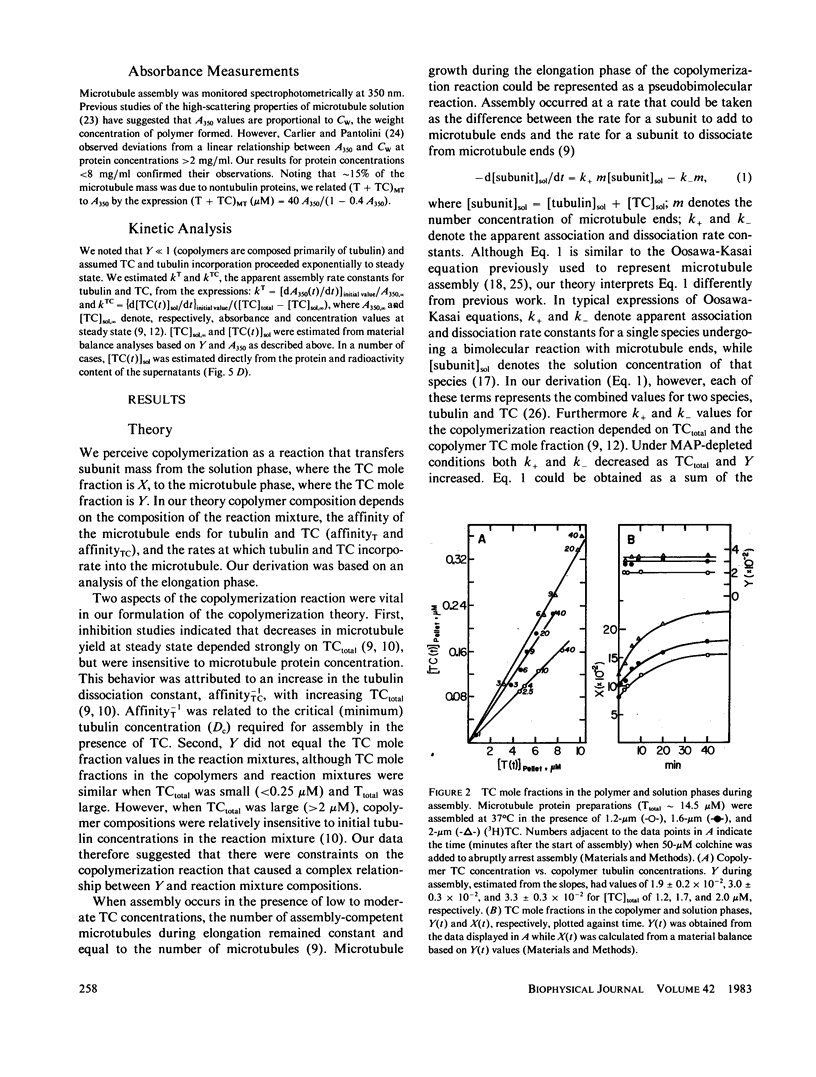
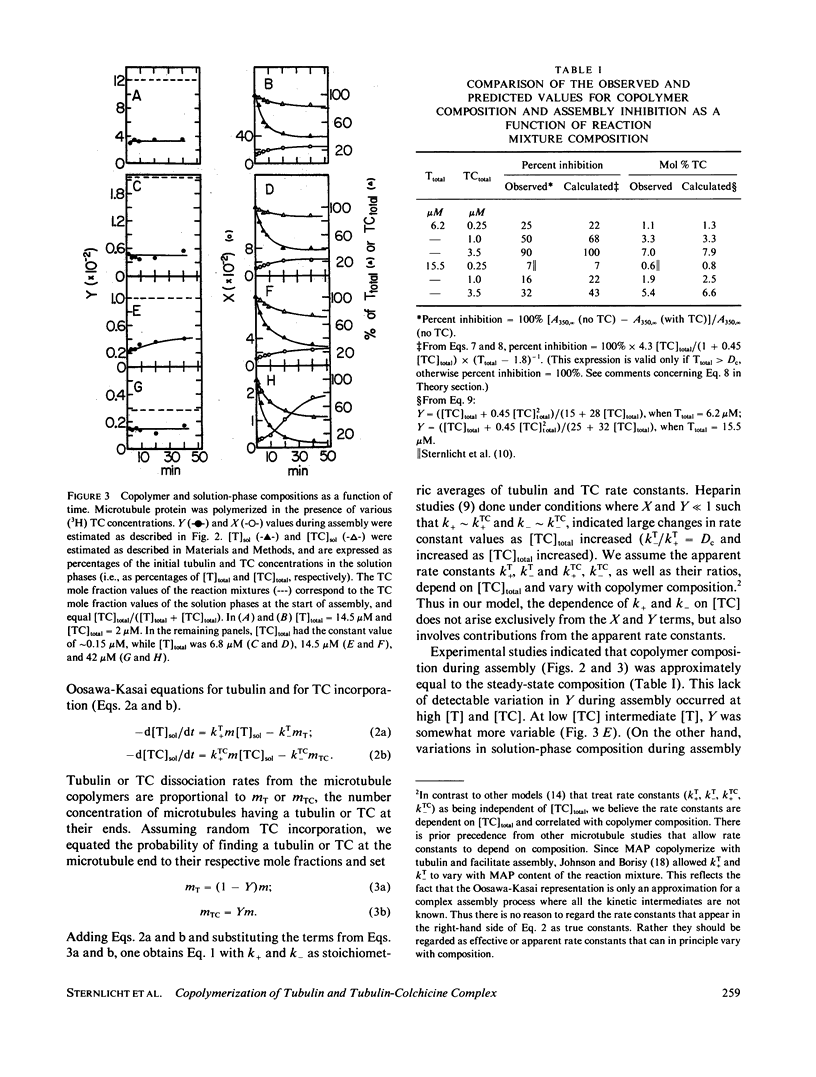
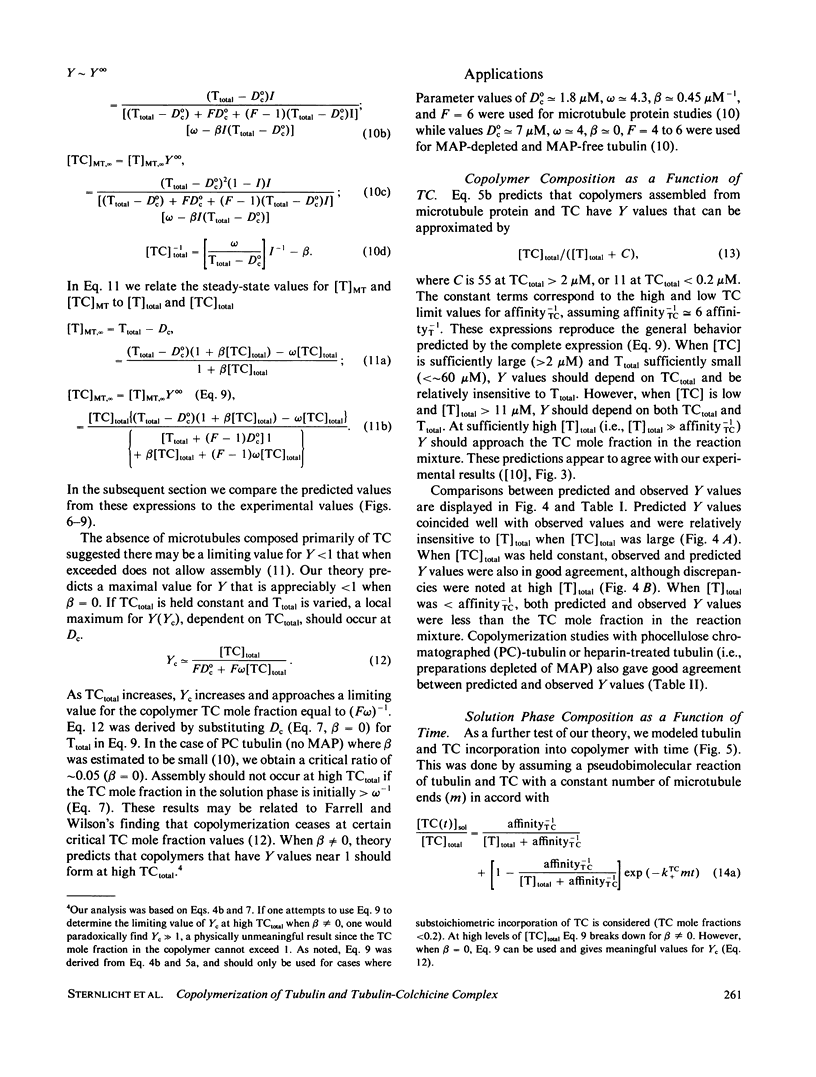
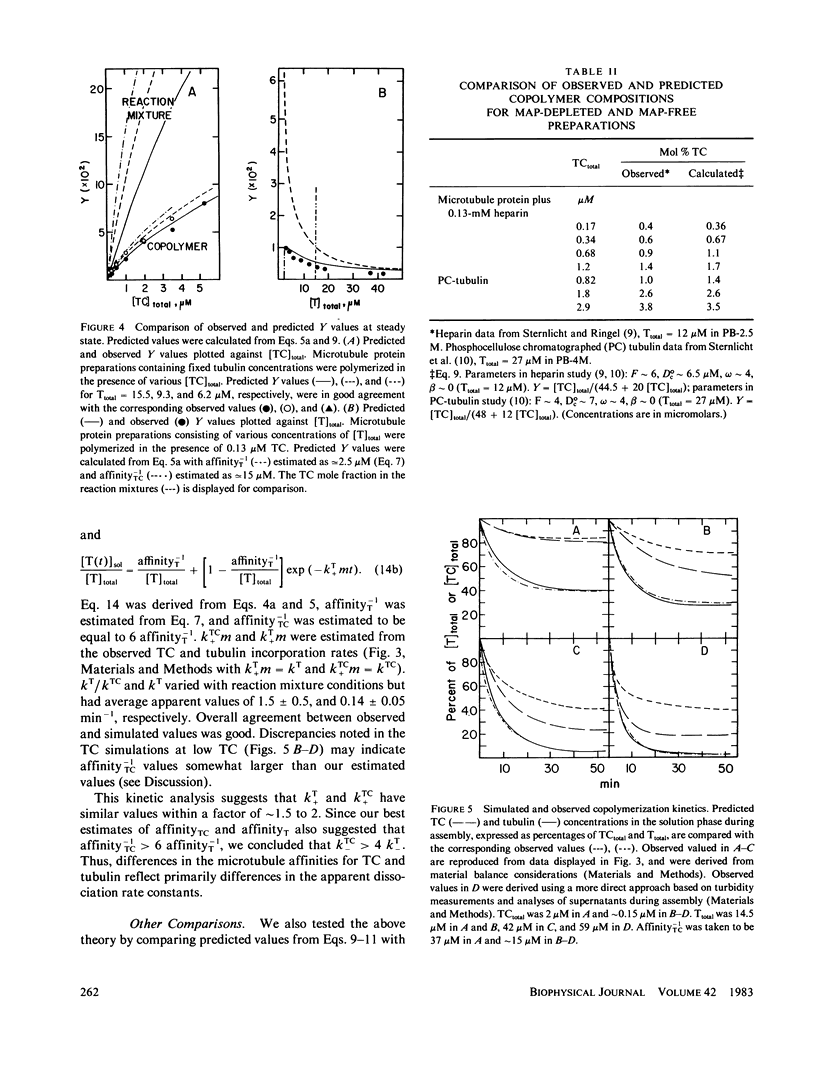
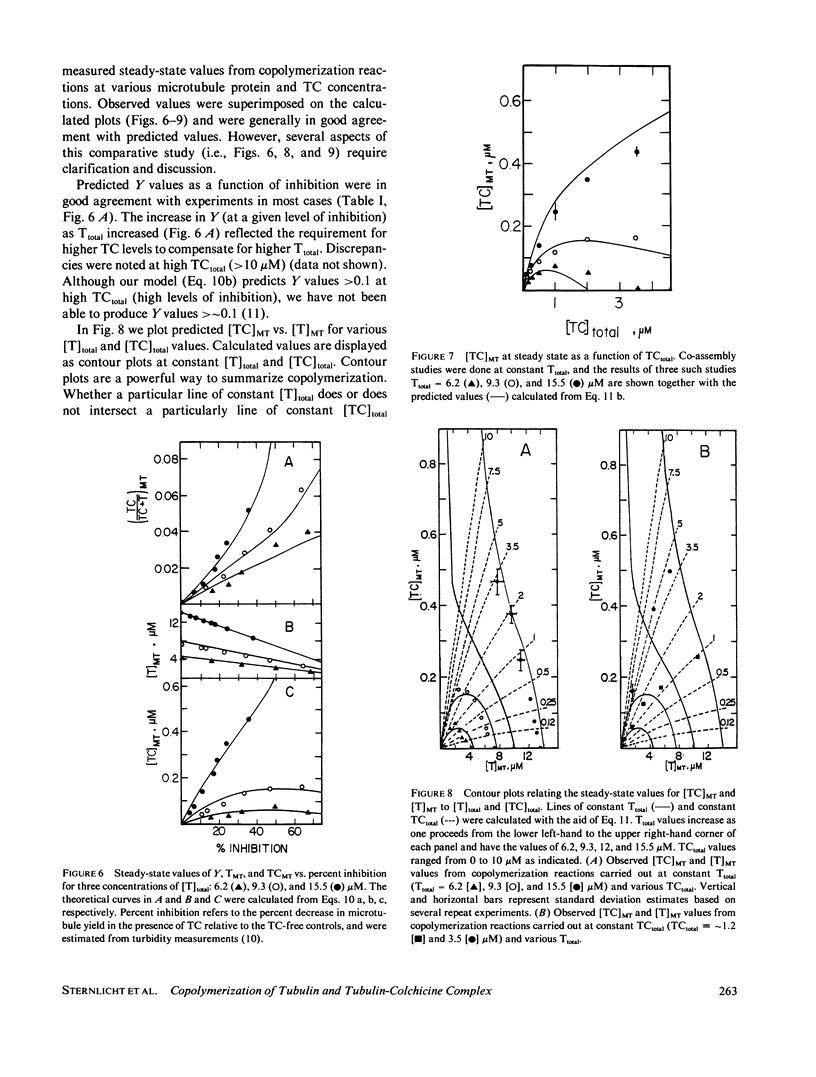
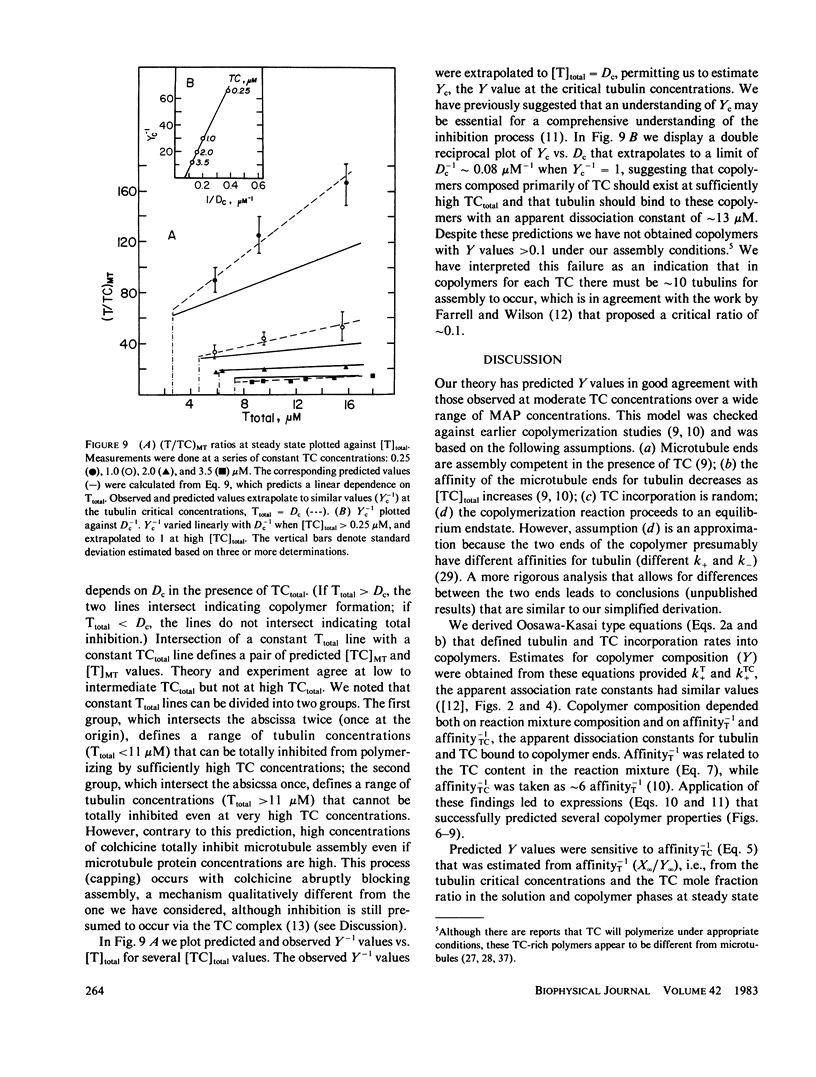
Substoichiometric concentrations of tubulin-colchicine complex (TC) inhibits microtubule assembly through a copolymerization reaction between tubulin and TC. We have determined the rates and extent of TC incorporation into bovine brain microtubules and developed a theory that models copolymerization. Our analysis suggests that while the apparent association rate constants for tubulin and TC are similar, the apparent dissociation rate constants for TC are a factor of five or more larger than those of tubulin. Copolymer composition showed only slight changes during assembly despite changes in the solution phase and showed little dependence at high TC upon the initial tubulin concentration. The theory was based on coupled Oosawa-Kasai equations that allow for the co-assembly of two components, tubulin and TC. An expression was derived that relates copolymer composition to reaction mixture composition and to the affinity of microtubule ends for tubulin and TC. This expression predicts copolymer composition at TC concentrations less than 10 microM and correlates composition with assembly inhibition. We perceive copolymerization as a facilitated incorporation of TC requiring the presence of tubulin. TC incorporation was dependent on the ratio of total tubulin to the dissociation constant for TC bound to microtubule ends. The copolymerization reaction is thus characterized by an interplay of two effects (a) where tubulin facilitates the incorporation of TC into the microtubule, and (b) where TC inhibits the assembly of tubulin into microtubules.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreu J. M., Timasheff S. N. Tubulin bound to colchicine forms polymers different from microtubules. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6753–6756. doi: 10.1073/pnas.79.22.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen L. G., Kuriyama R., Borisy G. G. Polarity of microtubules nucleated by centrosomes and chromosomes of Chinese hamster ovary cells in vitro. J Cell Biol. 1980 Jan;84(1):151–159. doi: 10.1083/jcb.84.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berne B. J. Interpretation of the light scattering from long rods. J Mol Biol. 1974 Nov 15;89(4):755–758. doi: 10.1016/0022-2836(74)90049-7. [DOI] [PubMed] [Google Scholar]
- Bryan J. A quantitative analysis of microtubule elongation. J Cell Biol. 1976 Dec;71(3):749–767. doi: 10.1083/jcb.71.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier M. F., Pantaloni D. Kinetic analysis of cooperativity in tubulin polymerization in the presence of guanosine di- or triphosphate nucleotides. Biochemistry. 1978 May 16;17(10):1908–1915. doi: 10.1021/bi00603a017. [DOI] [PubMed] [Google Scholar]
- Chambaut-Guérin A. M., Muller P., Rossignol B. Microtubules and protein secretion in rat lacrimal glands. Inhibitory effect of the tubulin . colchicine complex isolated from lacrimal glands upon brain tubulin polymerization. Identification of the complex by gel electrophoresis. J Biol Chem. 1979 Nov 10;254(21):10734–10739. [PubMed] [Google Scholar]
- Deery W. J., Weisenberg R. C. Kinetic and steady-state analysis of microtubules in the presence of colchicine. Biochemistry. 1981 Apr 14;20(8):2316–2324. doi: 10.1021/bi00511a038. [DOI] [PubMed] [Google Scholar]
- Farrell K. W., Wilson L. Proposed mechanism for colchicine poisoning of microtubules reassembled in vitro from Strongylocentrotus purpuratus sperm tail outer doublet tubulin. Biochemistry. 1980 Jun 24;19(13):3048–3054. doi: 10.1021/bi00554a033. [DOI] [PubMed] [Google Scholar]
- Gaskin F., Cantor C. R., Shelanski M. L. Turbidimetric studies of the in vitro assembly and disassembly of porcine neurotubules. J Mol Biol. 1974 Nov 15;89(4):737–755. doi: 10.1016/0022-2836(74)90048-5. [DOI] [PubMed] [Google Scholar]
- Johnson K. A., Borisy G. G. Kinetic analysis of microtubule self-assembly in vitro. J Mol Biol. 1977 Nov 25;117(1):1–31. doi: 10.1016/0022-2836(77)90020-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lambier A., Engelborghs Y. A quantitative analysis of tubulin-colchicine binding to microtubules. Eur J Biochem. 1980 Aug;109(2):619–624. doi: 10.1111/j.1432-1033.1980.tb04835.x. [DOI] [PubMed] [Google Scholar]
- Langford G. M. Arrangement of subunits in microtubules with 14 profilaments. J Cell Biol. 1980 Nov;87(2 Pt 1):521–526. doi: 10.1083/jcb.87.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malawista S. E. Microtubules and the mobilization of lysosomes in phagocytizing human leukocytes. Ann N Y Acad Sci. 1975 Jun 30;253:738–749. doi: 10.1111/j.1749-6632.1975.tb19242.x. [DOI] [PubMed] [Google Scholar]
- Margolis R. L., Rauch C. T., Wilson L. Mechanism of colchicine-dimer addition to microtubule ends: implications for the microtubule polymerization mechanism. Biochemistry. 1980 Nov 25;19(24):5550–5557. doi: 10.1021/bi00565a014. [DOI] [PubMed] [Google Scholar]
- Margolis R. L., Wilson L., Keifer B. I. Mitotic mechanism based on intrinsic microtubule behaviour. Nature. 1978 Mar 30;272(5652):450–452. doi: 10.1038/272450a0. [DOI] [PubMed] [Google Scholar]
- Margolis R. L., Wilson L. Opposite end assembly and disassembly of microtubules at steady state in vitro. Cell. 1978 Jan;13(1):1–8. doi: 10.1016/0092-8674(78)90132-0. [DOI] [PubMed] [Google Scholar]
- OOSAWA F., KASAI M. A theory of linear and helical aggregations of macromolecules. J Mol Biol. 1962 Jan;4:10–21. doi: 10.1016/s0022-2836(62)80112-0. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B., Borisy G. G. Characterization of microtubule assembly in porcine brain extracts by viscometry. Biochemistry. 1973 Oct 9;12(21):4282–4289. doi: 10.1021/bi00745a037. [DOI] [PubMed] [Google Scholar]
- Oppenheim D. S., Hauschka B. T., McIntosh J. R. Anaphase motions in dilute colchicine. Evidence of two phases in chromosome segregation. Exp Eye Res. 1973 Apr;79(1):95–105. [PubMed] [Google Scholar]
- Reaven E. P., Reaven G. M. A quantitative ultrastructural study of microtubule content and secretory granule accumulation in parathyroid glands of phosphate- and colchicine-treated rats. J Clin Invest. 1975 Jul;56(1):49–55. doi: 10.1172/JCI108078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval I. V., Weber K. Polymerization of tubulin in the presence of colchicine or podophyllotoxin. Formation of a ribbon structure induced by guanylyl-5'-methylene diphosphonate. J Mol Biol. 1979 Oct 15;134(1):159–172. doi: 10.1016/0022-2836(79)90418-2. [DOI] [PubMed] [Google Scholar]
- Schmitt H., Atlas D. Specific affinity labelling of tubulin with bromocolchicine. J Mol Biol. 1976 Apr 25;102(4):743–758. doi: 10.1016/0022-2836(76)90289-8. [DOI] [PubMed] [Google Scholar]
- Sternlicht H., Ringel I. Colchicine inhibition of microtubule assembly via copolymer formation. J Biol Chem. 1979 Oct 25;254(20):10540–10550. [PubMed] [Google Scholar]
- Sternlicht H., Ringel I., Szasz J. A kinetic model for colchicine inhibition of microtubule assembly. Biophys J. 1980 Oct;32(1):445–448. doi: 10.1016/S0006-3495(80)84976-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht H., Ringel I., Szasz J. The co-polymerization of tubulin and tubulin chochicine complex in the absence and presence of associated proteins. J Biol Chem. 1980 Oct 10;255(19):9138–9148. [PubMed] [Google Scholar]
- Wilson L. Properties of colchicine binding protein from chick embryo brain. Interactions with vinca alkaloids and podophyllotoxin. Biochemistry. 1970 Dec 8;9(25):4999–5007. doi: 10.1021/bi00827a026. [DOI] [PubMed] [Google Scholar]
- Zackroff R. V., Weisenberg R. C., Deery W. J. Equilibrium and kinetic analysis of microtubule assembly in the presence of guanosine diphosphate. J Mol Biol. 1980 Jun 5;139(4):641–659. doi: 10.1016/0022-2836(80)90053-4. [DOI] [PubMed] [Google Scholar]