Abstract
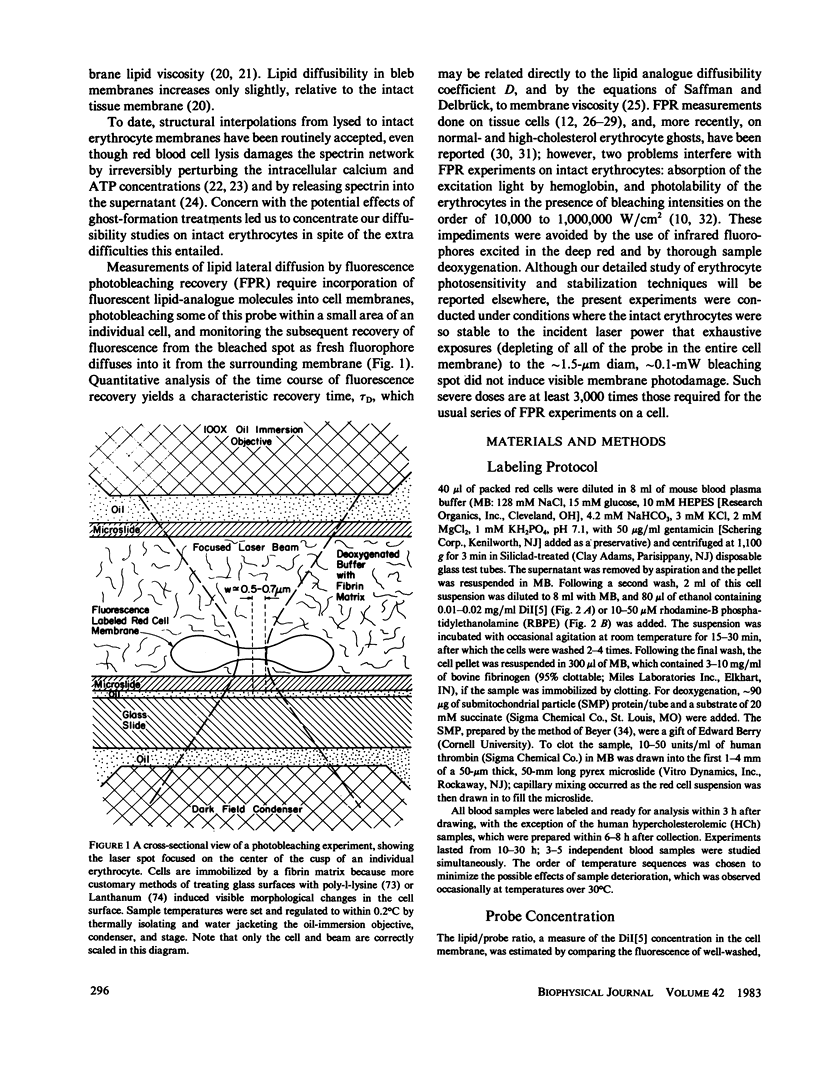
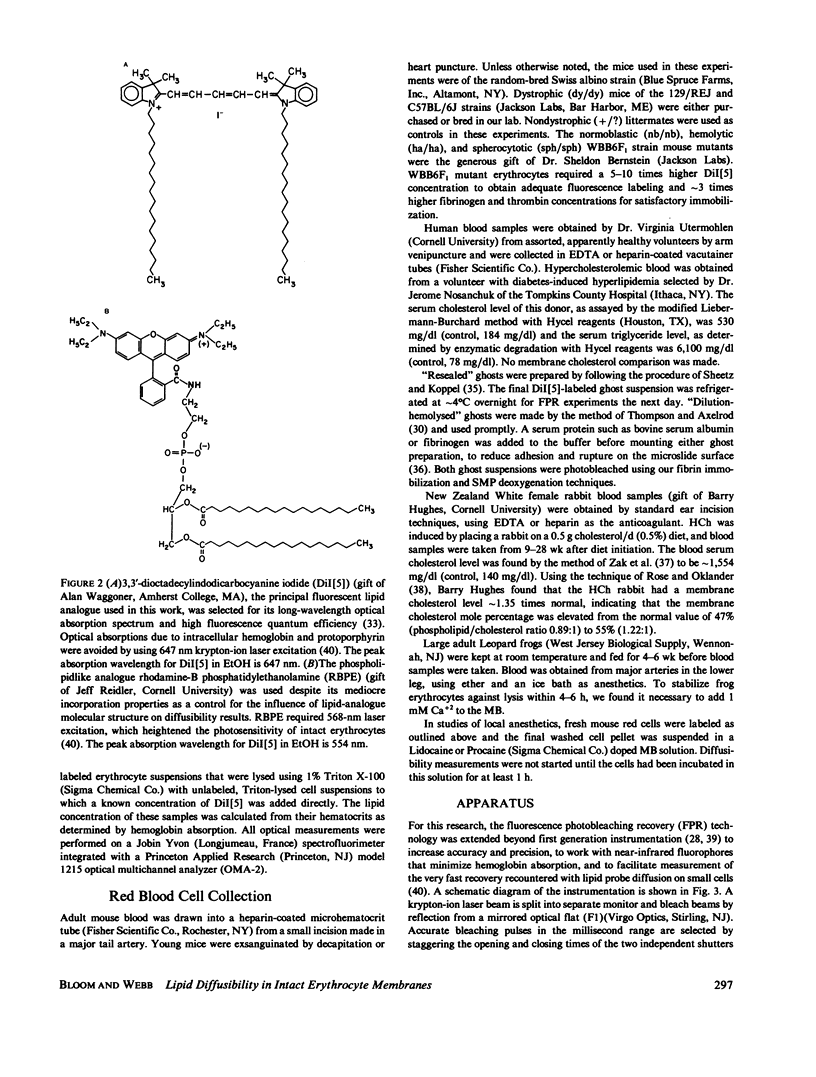
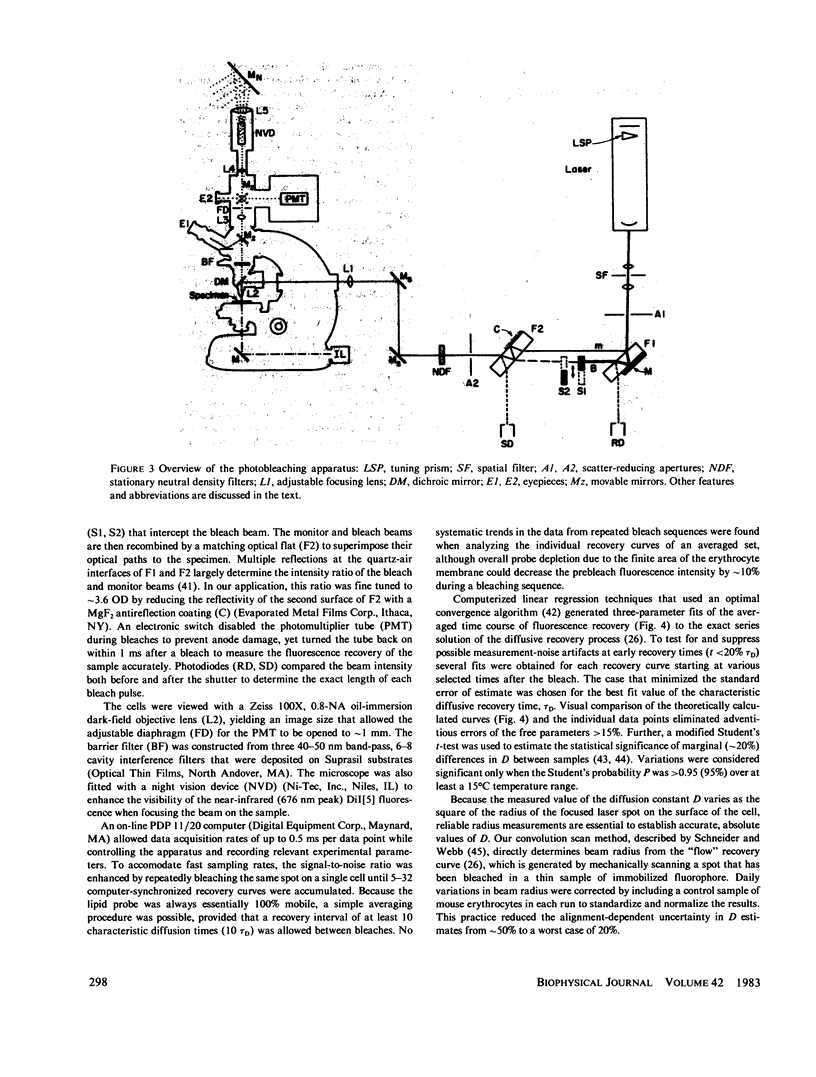
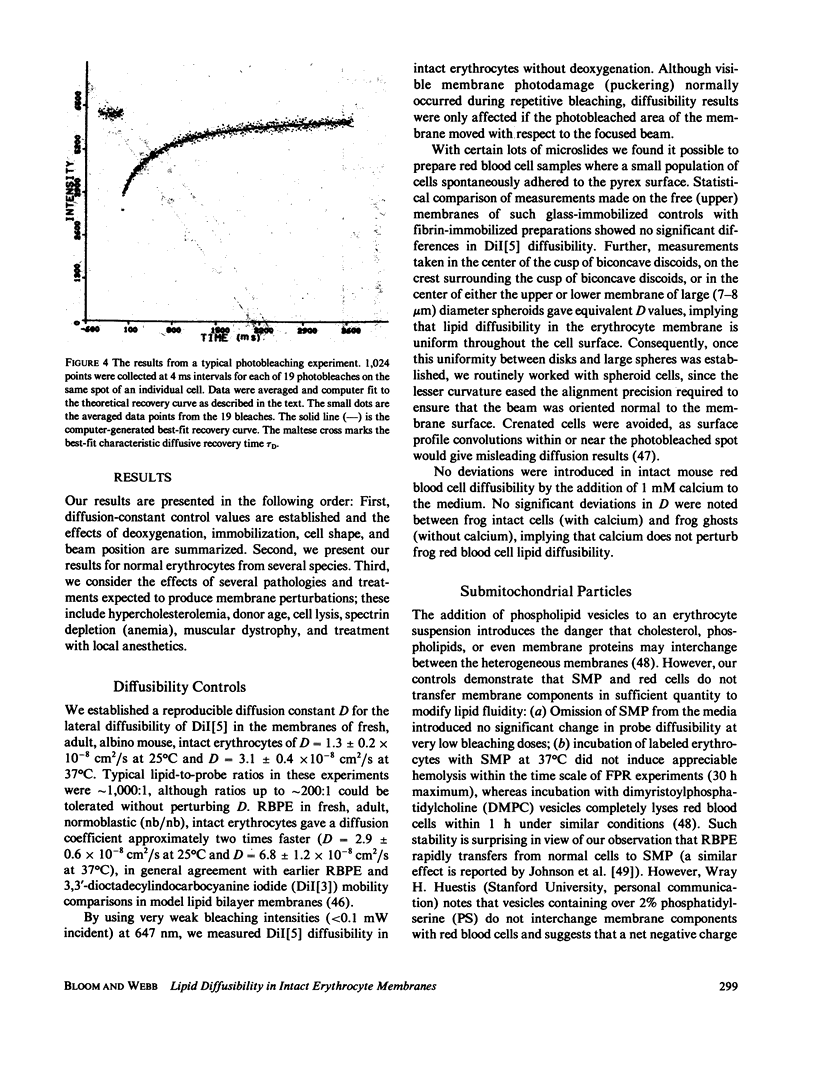
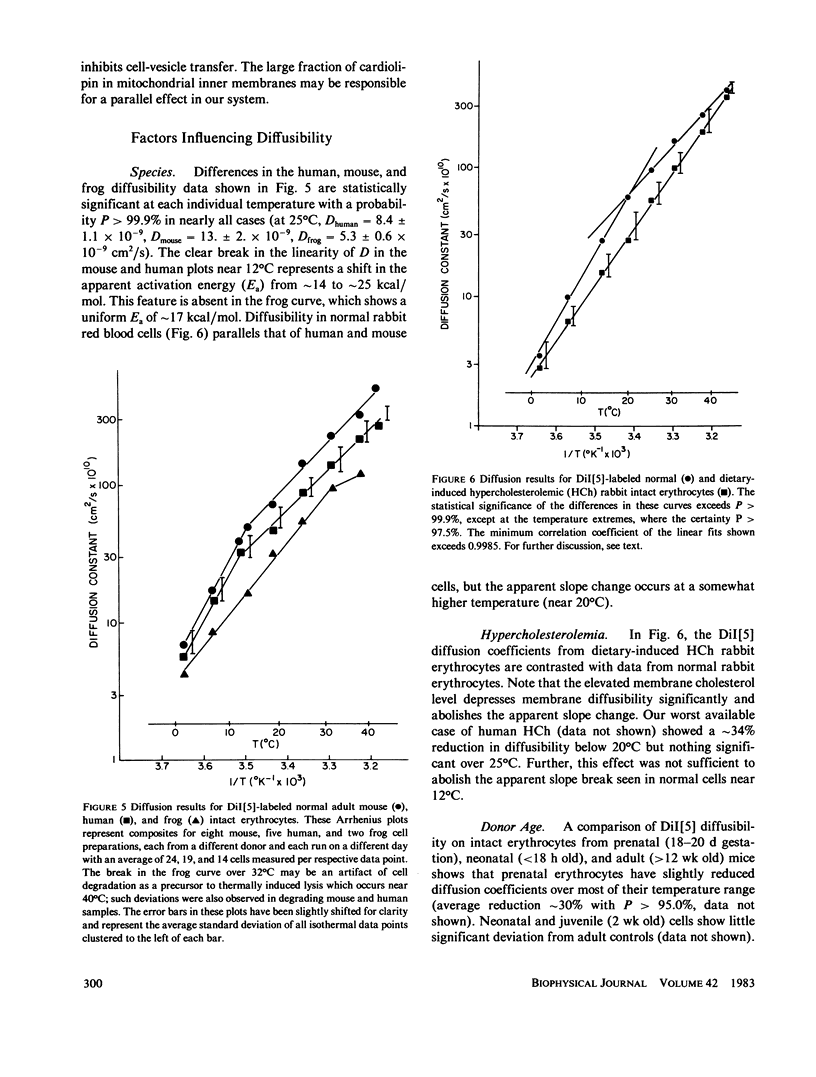
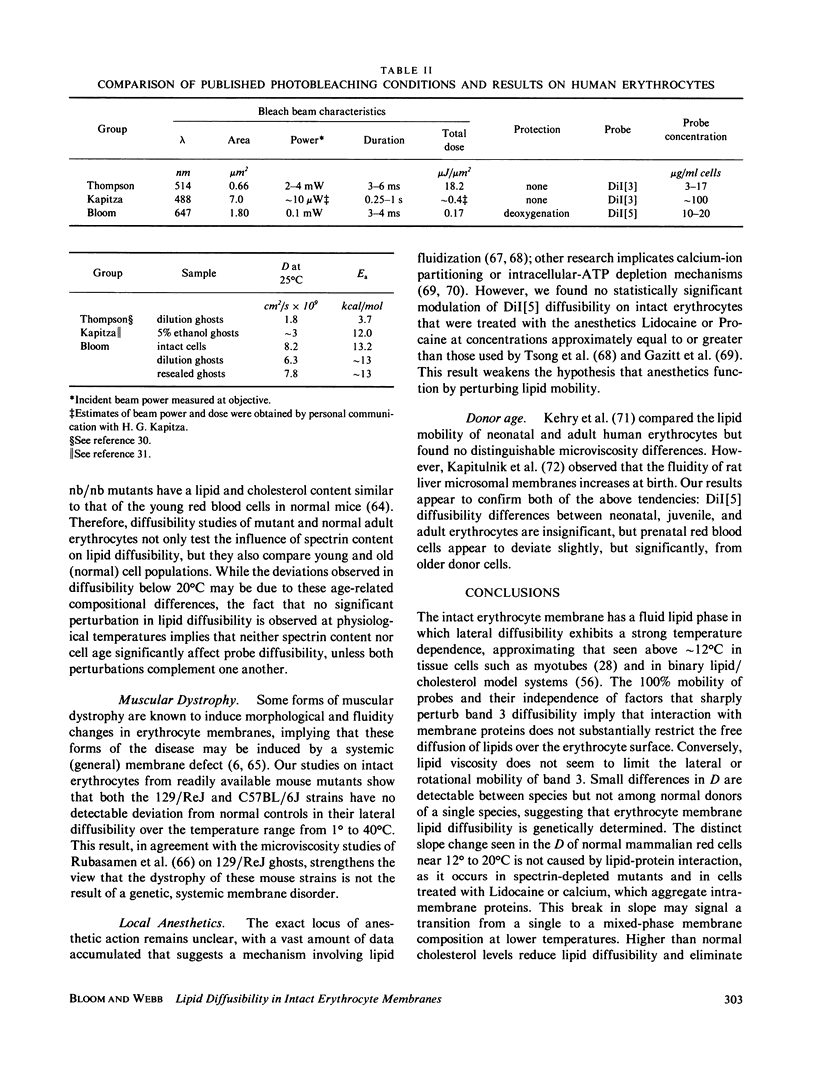
The lateral diffusion of fluorescent lipid analogues in the plasma membrane of intact erythrocytes from man, mouse, rabbit, and frog has been measured by fluorescence photobleaching recovery (FPR). Intact cells from dystrophic, normoblastic, hemolytic, and spherocytotic mouse mutants; from hypercholesterolemic rabbits and humans; and from prenatal, neonatal, and juvenile mice have been compared with corresponding normals. The lateral diffusion coefficient (D) for 3,3'-dioctadecylindodicarbocyanine iodide (DiI[5]) in intact normal human erythrocytes is D = 8.2 +/- 1.2 X 10(-9) cm2/s at 25 degrees C and D = 2.1 +/- 0.4 X 10(-8) cm2/s at 37 degrees C, and varies approximately 50-fold between 1 degree and 42 degrees C. The diffusion constants of lipid analogue rhodamine-B phosphatidylethanolamine (RBPE) are about twice those of DiI[5]. The temperature dependence and magnitude of D vary by up to a factor of 3 between species and are only influenced by donor age in prenatals. DiI[5] diffusibility is not perturbed by the presence of calcium or local anesthetics or by spectrin depletion (via mutation). However, lipid-analogue diffusibility in erythrocyte ghosts may differ from intact cells. Dietary hypercholesterolemia in rabbits reduces the diffusion coefficient and eliminates the characteristic break in Arrhenius plots of D found in all other cells studied except frog.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D. Cell surface heating during fluorescence photobleaching recovery experiments. Biophys J. 1977 Apr;18(1):129–131. doi: 10.1016/S0006-3495(77)85601-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D., Wight A., Webb W., Horwitz A. Influence of membrane lipids on acetylcholine receptor and lipid probe diffusion in cultured myotube membrane. Biochemistry. 1978 Aug 22;17(17):3604–3609. doi: 10.1021/bi00610a029. [DOI] [PubMed] [Google Scholar]
- Bernstein S. E. Inherited hemolytic disease in mice: a review and update. Lab Anim Sci. 1980 Apr;30(2 Pt 1):197–205. [PubMed] [Google Scholar]
- Brecher G., Bessis M. Present status of spiculed red cells and their relationship to the discocyte-echinocyte transformation: a critical review. Blood. 1972 Sep;40(3):333–344. [PubMed] [Google Scholar]
- Cherry R. J. Rotational and lateral diffusion of membrane proteins. Biochim Biophys Acta. 1979 Dec 20;559(4):289–327. doi: 10.1016/0304-4157(79)90009-1. [DOI] [PubMed] [Google Scholar]
- Clarke M., Schatten G., Mazia D., Spudich J. A. Visualization of actin fibers associated with the cell membrane in amoebae of Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1975 May;72(5):1758–1762. doi: 10.1073/pnas.72.5.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. A., Durocher J. R., Leslie M. H. Decreased fluidity of red cell membrane lipids in abetalipoproteinemia. J Clin Invest. 1977 Jul;60(1):115–121. doi: 10.1172/JCI108747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. A., Leslie M. H., Fischkoff S., Shinitzky M., Shattil S. J. Factors influencing the lipid composition and fluidity of red cell membranes in vitro: production of red cells possessing more than two cholesterols per phospholipid. Biochemistry. 1978 Jan 24;17(2):327–331. doi: 10.1021/bi00595a021. [DOI] [PubMed] [Google Scholar]
- Cullis P. R. Hydrocarbon phase transitions, heterogeneous lipid distributions and lipid-protein interactions in erythrocyte membranes. FEBS Lett. 1976 Oct 1;68(2):173–176. doi: 10.1016/0014-5793(76)80430-9. [DOI] [PubMed] [Google Scholar]
- Evans E. A., Hochmuth R. M. A solid-liquid composite model of the red cell membrane. J Membr Biol. 1977 Jan 28;30(4):351–362. doi: 10.1007/BF01869676. [DOI] [PubMed] [Google Scholar]
- Fahey P. F., Koppel D. E., Barak L. S., Wolf D. E., Elson E. L., Webb W. W. Lateral diffusion in planar lipid bilayers. Science. 1977 Jan 21;195(4275):305–306. doi: 10.1126/science.831279. [DOI] [PubMed] [Google Scholar]
- Fahey P. F., Webb W. W. Lateral diffusion in phospholipid bilayer membranes and multilamellar liquid crystals. Biochemistry. 1978 Jul 25;17(15):3046–3053. doi: 10.1021/bi00608a016. [DOI] [PubMed] [Google Scholar]
- Feinstein M. B., Fernandez S. M., Sha'afi R. I. Fluidity of natural membranes and phosphatidylserine and ganglioside dispersions. Effect of local anesthetics, cholesterol and protein. Biochim Biophys Acta. 1975 Dec 16;413(3):354–370. doi: 10.1016/0005-2736(75)90121-2. [DOI] [PubMed] [Google Scholar]
- Fowler V., Branton D. Lateral mobility of human erythrocyte integral membrane proteins. Nature. 1977 Jul 7;268(5615):23–26. doi: 10.1038/268023a0. [DOI] [PubMed] [Google Scholar]
- Gazitt Y., Loyter A., Ohad I. Induction of ATP depletion, intramembrane particle aggregation and exposure of membrane phospholipids in chicken erythrocytes by local anesthetics and tranquilizers. Biochim Biophys Acta. 1977 Dec 15;471(3):361–371. doi: 10.1016/0005-2736(77)90042-6. [DOI] [PubMed] [Google Scholar]
- Golan D. E., Veatch W. Lateral mobility of band 3 in the human erythrocyte membrane studied by fluorescence photobleaching recovery: evidence for control by cytoskeletal interactions. Proc Natl Acad Sci U S A. 1980 May;77(5):2537–2541. doi: 10.1073/pnas.77.5.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis W. H. A sodium-specific membrane permeability defect induced by phospholipid vesicle treatment of erythrocytes. J Biol Chem. 1977 Oct 10;252(19):6764–6768. [PubMed] [Google Scholar]
- Jacobson K., Hou Y., Wojcieszyn J. Evidence for lack of damage during photobleaching measurements of the lateral mobility of cell surface components. Exp Cell Res. 1978 Oct 1;116(1):179–189. doi: 10.1016/0014-4827(78)90074-5. [DOI] [PubMed] [Google Scholar]
- Johnson L. V., Walsh M. L., Chen L. B. Localization of mitochondria in living cells with rhodamine 123. Proc Natl Acad Sci U S A. 1980 Feb;77(2):990–994. doi: 10.1073/pnas.77.2.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansu E., Krasnow S. H., Ballas S. K. Spectrin loss during in vitro red cell lysis. Biochim Biophys Acta. 1980 Feb 15;596(1):18–27. doi: 10.1016/0005-2736(80)90167-4. [DOI] [PubMed] [Google Scholar]
- Kapitulnik J., Tshershedsky M., Barenholz Y. Fluidity of the rat liver microsomal membrane: increase at birth. Science. 1979 Nov 16;206(4420):843–844. doi: 10.1126/science.493984. [DOI] [PubMed] [Google Scholar]
- Kapitza H. G., Sackmann E. Local measurement of lateral motion in erythrocyte membranes by photobleaching technique. Biochim Biophys Acta. 1980;595(1):56–64. doi: 10.1016/0005-2736(80)90247-3. [DOI] [PubMed] [Google Scholar]
- Kehry M., Yguerabide J., Singer S. J. Fluidity in the membranes of adult and neonatal human erythrocytes. Science. 1977 Feb 4;195(4277):486–487. doi: 10.1126/science.835005. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick F. Spectrin: current understanding of its physical, biochemical, and functional properties. Life Sci. 1976 Jul 1;19(1):1–17. doi: 10.1016/0024-3205(76)90368-4. [DOI] [PubMed] [Google Scholar]
- Koppel D. E., Axelrod D., Schlessinger J., Elson E. L., Webb W. W. Dynamics of fluorescence marker concentration as a probe of mobility. Biophys J. 1976 Nov;16(11):1315–1329. doi: 10.1016/S0006-3495(76)85776-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel D. E. Fluorescence redistribution after photobleaching. A new multipoint analysis of membrane translational dynamics. Biophys J. 1979 Nov;28(2):281–291. doi: 10.1016/S0006-3495(79)85176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel D. E., Sheetz M. P., Schindler M. Lateral diffusion in biological membranes. A normal-mode analysis of diffusion on a spherical surface. Biophys J. 1980 Apr;30(1):187–192. doi: 10.1016/S0006-3495(80)85087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel D. E., Sheetz M. P., Schindler M. Matrix control of protein diffusion in biological membranes. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3576–3580. doi: 10.1073/pnas.78.6.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz B. R., Barrow D. A., Hoechli M. Cholesterol-phosphatidylcholine interactions in multilamellar vesicles. Biochemistry. 1980 Apr 29;19(9):1943–1954. doi: 10.1021/bi00550a034. [DOI] [PubMed] [Google Scholar]
- Lorand L., Siefring G. E., Jr, Lowe-Krentz L. Formation of gamma-glutamyl-epsilon-lysine bridges between membrane proteins by a Ca2+-regulated enzyme in intact erythrocytes. J Supramol Struct. 1978;9(3):427–440. doi: 10.1002/jss.400090313. [DOI] [PubMed] [Google Scholar]
- Lux S. E., Pease B., Tomaselli M. B., John K. M., Bernstein S. E. Hemolytic anemias associated with deficient or dysfunctional spectrin. Prog Clin Biol Res. 1979;30:463–469. [PubMed] [Google Scholar]
- Maeda T., Eldridge C., Toyama S., Ohnishi S. I., Elson E. L., Webb W. W. Membrane receptor mobility changes by Sendai virus. Exp Cell Res. 1979 Oct 15;123(2):333–343. doi: 10.1016/0014-4827(79)90475-0. [DOI] [PubMed] [Google Scholar]
- Magde D., Elson E. L., Webb W. W. Fluorescence correlation spectroscopy. II. An experimental realization. Biopolymers. 1974 Jan;13(1):29–61. doi: 10.1002/bip.1974.360130103. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Furthmayr H., Tomita M. The red cell membrane. Annu Rev Biochem. 1976;45:667–698. doi: 10.1146/annurev.bi.45.070176.003315. [DOI] [PubMed] [Google Scholar]
- Matheson D. W., Howland J. L. Erythrocyte deformation in human muscular dystrophy. Science. 1974 Apr 12;184(4133):165–166. doi: 10.1126/science.184.4133.165. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Cell shape changes and transmembrane receptor uncoupling induced by tertiary amine local anesthetic. J Supramol Struct. 1976;5(1):65–72. doi: 10.1002/jss.400050107. [DOI] [PubMed] [Google Scholar]
- Nigg E. A., Cherry R. J. Influence of temperature and cholesterol on the rotational diffusion of band 3 in the human erythrocyte membrane. Biochemistry. 1979 Aug 7;18(16):3457–3465. doi: 10.1021/bi00583a004. [DOI] [PubMed] [Google Scholar]
- Nigg E., Kessler M., Cherry R. J. Labeling of human erythrocyte membranes with eosin probes used for protein diffusion measurements: inhibition of anion transport and photo-oxidative inactivation of acetylcholinesterase. Biochim Biophys Acta. 1979 Jan 19;550(2):328–340. doi: 10.1016/0005-2736(79)90219-0. [DOI] [PubMed] [Google Scholar]
- Peters R., Peters J., Tews K. H., Bähr W. A microfluorimetric study of translational diffusion in erythrocyte membranes. Biochim Biophys Acta. 1974 Nov 15;367(3):282–294. doi: 10.1016/0005-2736(74)90085-6. [DOI] [PubMed] [Google Scholar]
- ROSE H. G., OKLANDER M. IMPROVED PROCEDURE FOR THE EXTRACTION OF LIPIDS FROM HUMAN ERYTHROCYTES. J Lipid Res. 1965 Jul;6:428–431. [PubMed] [Google Scholar]
- Rubenstein J. L., Smith B. A., McConnell H. M. Lateral diffusion in binary mixtures of cholesterol and phosphatidylcholines. Proc Natl Acad Sci U S A. 1979 Jan;76(1):15–18. doi: 10.1073/pnas.76.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubsamen H., Barald P., Podleski T. A specific decrease of the fluorescence depolarization of perylene in muscle membranes from mice with muscular dystrophy. Biochim Biophys Acta. 1976 Dec 14;455(3):767–779. doi: 10.1016/0005-2736(76)90047-x. [DOI] [PubMed] [Google Scholar]
- Saffman P. G., Delbrück M. Brownian motion in biological membranes. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3111–3113. doi: 10.1073/pnas.72.8.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schekman R., Singer S. J. Clustering and endocytosis of membrane receptors can be induced in mature erythrocytes of neonatal but not adult humans. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4075–4079. doi: 10.1073/pnas.73.11.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler M., Koppel D. E., Sheetz M. P. Modulation of membrane protein lateral mobility by polyphosphates and polyamines. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1457–1461. doi: 10.1073/pnas.77.3.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Koppel D. E., Axelrod D., Jacobson K., Webb W. W., Elson E. L. Lateral transport on cell membranes: mobility of concanavalin A receptors on myoblasts. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2409–2413. doi: 10.1073/pnas.73.7.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schothorst A. A., Van Steveninck J., Went L. N., Suurmond D. Protoporphyrin-induced photohemolysis in protoporphyria and in normal red blood cells. Clin Chim Acta. 1970 Apr;28(1):41–49. doi: 10.1016/0009-8981(70)90158-0. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Koppel D. E. Membrane damage caused by irradiation of fluorescent concanavalin A. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3314–3317. doi: 10.1073/pnas.76.7.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinitzky M., Barenholz Y. Fluidity parameters of lipid regions determined by fluorescence polarization. Biochim Biophys Acta. 1978 Dec 15;515(4):367–394. doi: 10.1016/0304-4157(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Sims P. J., Waggoner A. S., Wang C. H., Hoffman J. F. Studies on the mechanism by which cyanine dyes measure membrane potential in red blood cells and phosphatidylcholine vesicles. Biochemistry. 1974 Jul 30;13(16):3315–3330. doi: 10.1021/bi00713a022. [DOI] [PubMed] [Google Scholar]
- Smith D. K., Palek J. Modulation of lateral mobility of band 3 in the red cell membrane by oxidative cross-linking of spectrin. Nature. 1982 Jun 3;297(5865):424–425. doi: 10.1038/297424a0. [DOI] [PubMed] [Google Scholar]
- Smith T. C., Levinson C. Direct measurement of the membrane potential of Ehrlich ascites tumor cells: lack of effect of valinomycin and ouabain. J Membr Biol. 1975;23(3-4):349–365. doi: 10.1007/BF01870257. [DOI] [PubMed] [Google Scholar]
- Tanaka K. I., Ohnishi S. Heterogeneity in the fluidity of intact erythrocyte membrane and its homogenization upon hemolysis. Biochim Biophys Acta. 1976 Mar 5;426(2):218–231. doi: 10.1016/0005-2736(76)90333-3. [DOI] [PubMed] [Google Scholar]
- Tank D. W., Wu E. S., Webb W. W. Enhanced molecular diffusibility in muscle membrane blebs: release of lateral constraints. J Cell Biol. 1982 Jan;92(1):207–212. doi: 10.1083/jcb.92.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson N. L., Axelrod D. Reduced lateral mobility of a fluorescent lipid probe in cholesterol-depleted erythrocyte membrane. Biochim Biophys Acta. 1980 Mar 27;597(1):155–165. doi: 10.1016/0005-2736(80)90159-5. [DOI] [PubMed] [Google Scholar]
- Tsong T. Y., Greenberg M., Kanehisa M. I. Anesthetic action of membrane lipids. Biochemistry. 1977 Jul 12;16(14):3115–3121. doi: 10.1021/bi00633a012. [DOI] [PubMed] [Google Scholar]
- Verma S. P., Wallach D. F. Erythrocyte membranes undergo cooperative, pH-sensitive state transitions in the physiological temperature range: evidence from Raman spectroscopy. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3558–3561. doi: 10.1073/pnas.73.10.3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S. P., Wallach D. F. Multiple thermotropic state transitions in erythrocyte membranes. A laser-Raman study of the CH-stretching and acoustical regions. Biochim Biophys Acta. 1976 Jun 17;436(2):307–318. doi: 10.1016/0005-2736(76)90196-6. [DOI] [PubMed] [Google Scholar]
- Webb W. W., Barak L. S., Tank D. W., Wu E. S. Molecular mobility on the cell surface. Biochem Soc Symp. 1981;(46):191–205. [PubMed] [Google Scholar]


