Abstract
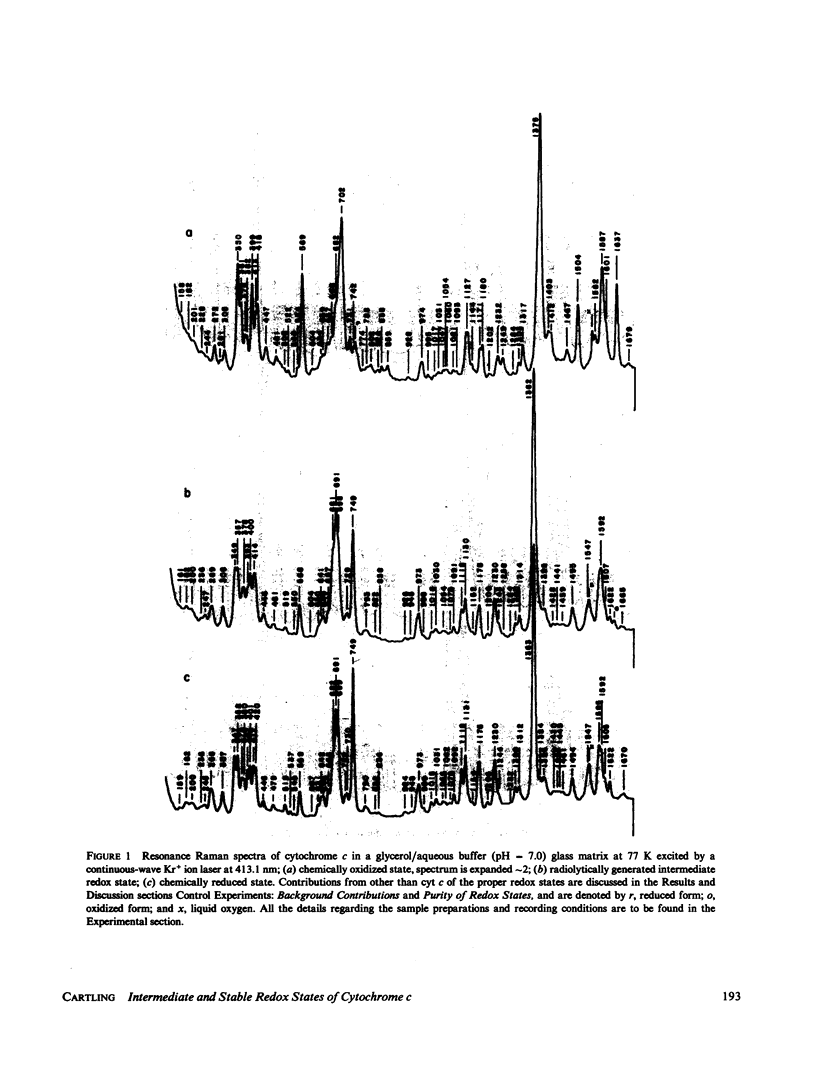
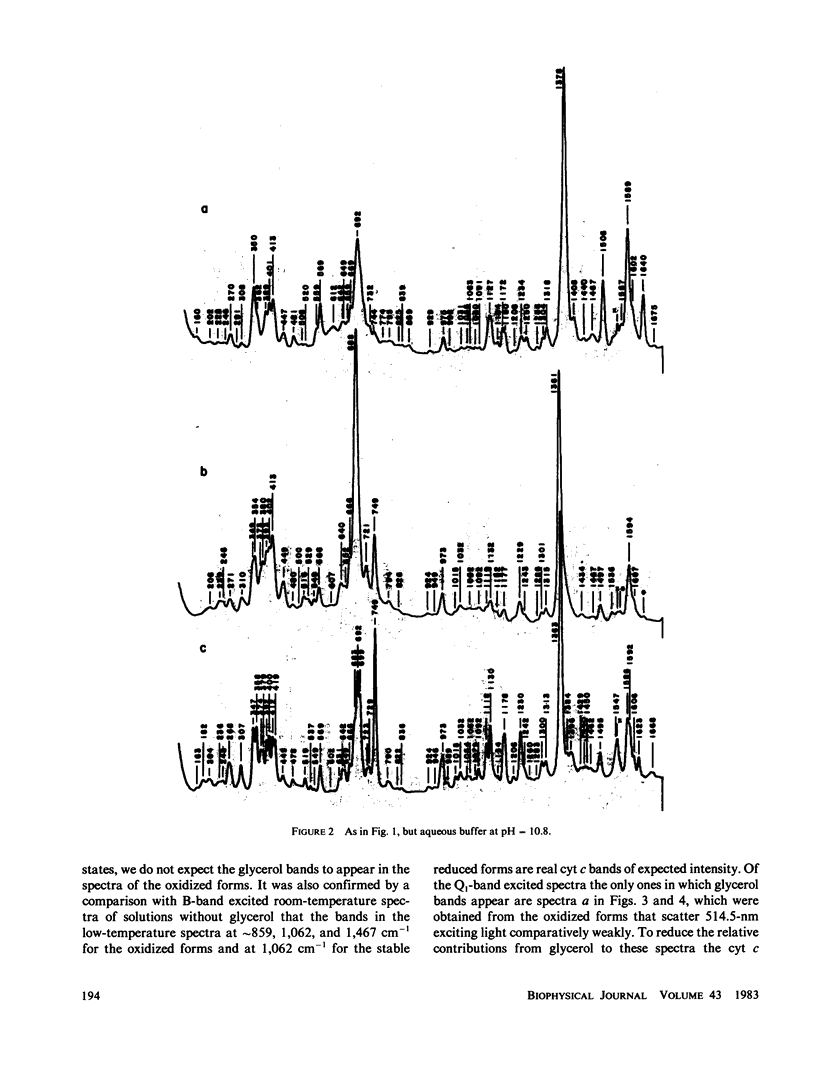
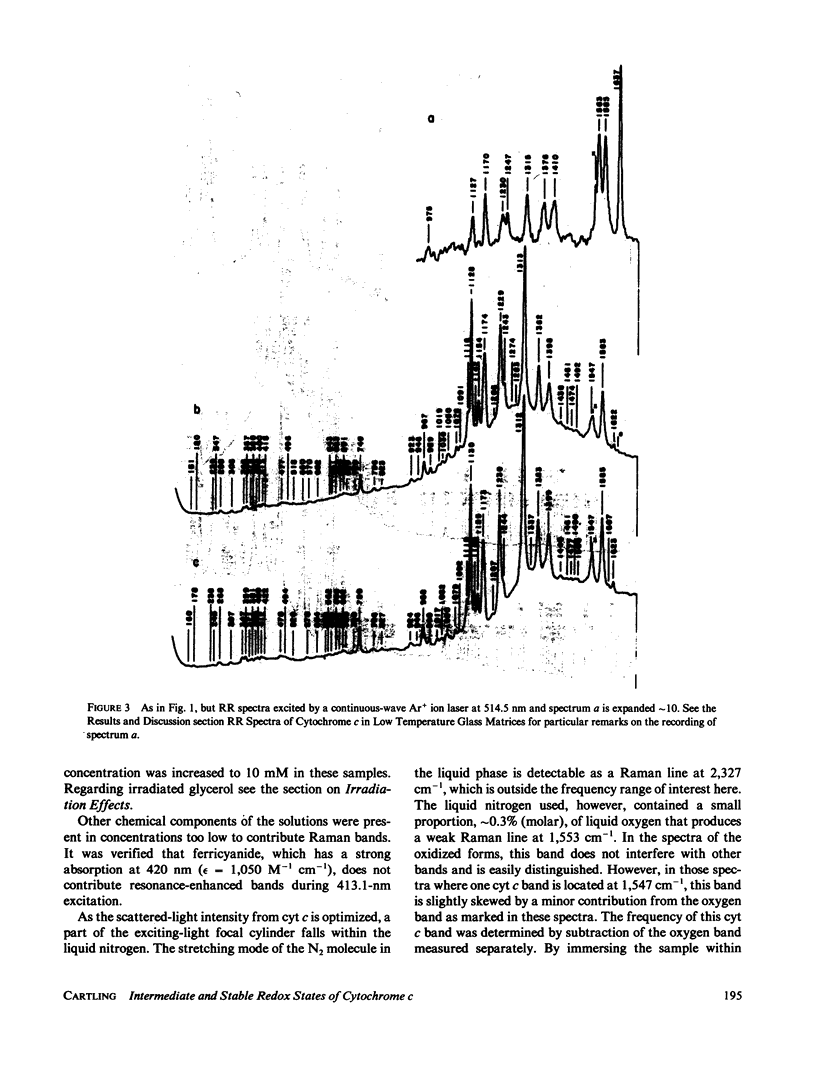
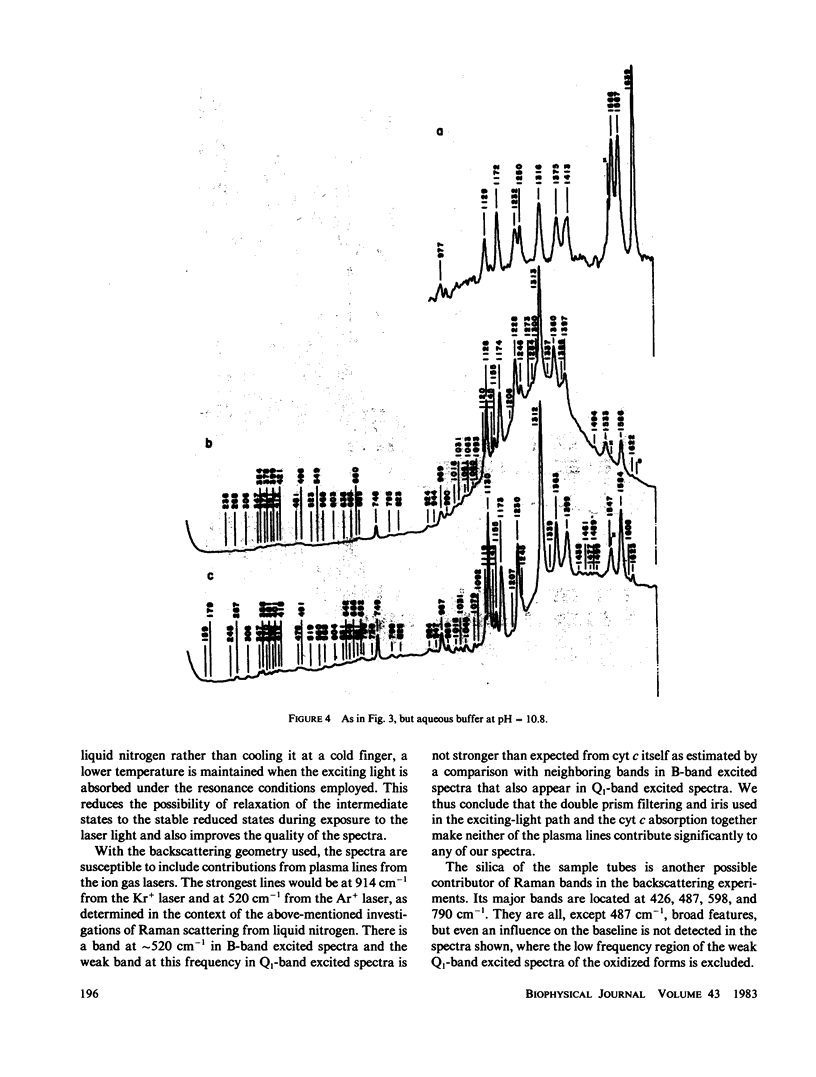
Stabilized intermediate redox states of cytochrome c are generated by radiolytic reduction of initially oxidized enzyme in glass matrices at liquid nitrogen temperature. In the intermediate states the heme group is reduced by hydrated electrons, whereas the protein conformation is restrained close to its oxidized form by the low-temperature glass matrix. The intermediate and stable redox states of cytochrome c at neutral and alkaline pH are studied by low-temperature resonance Raman spectroscopy using excitations in resonance with the B (Soret) and Q1 (beta) optical transitions. The assignments of the cytochrome c resonance Raman bands are discussed. The observed spectral characteristics of the intermediate states as well as of the alkaline transition in the oxidized state are interpreted in terms of oxidation-state marker modes, spin-state marker modes, heme iron--axial ligand stretching modes, totally symmetric in-plane porphyrin modes, nontotally symmetric in-plane modes, and out-of-plane modes.
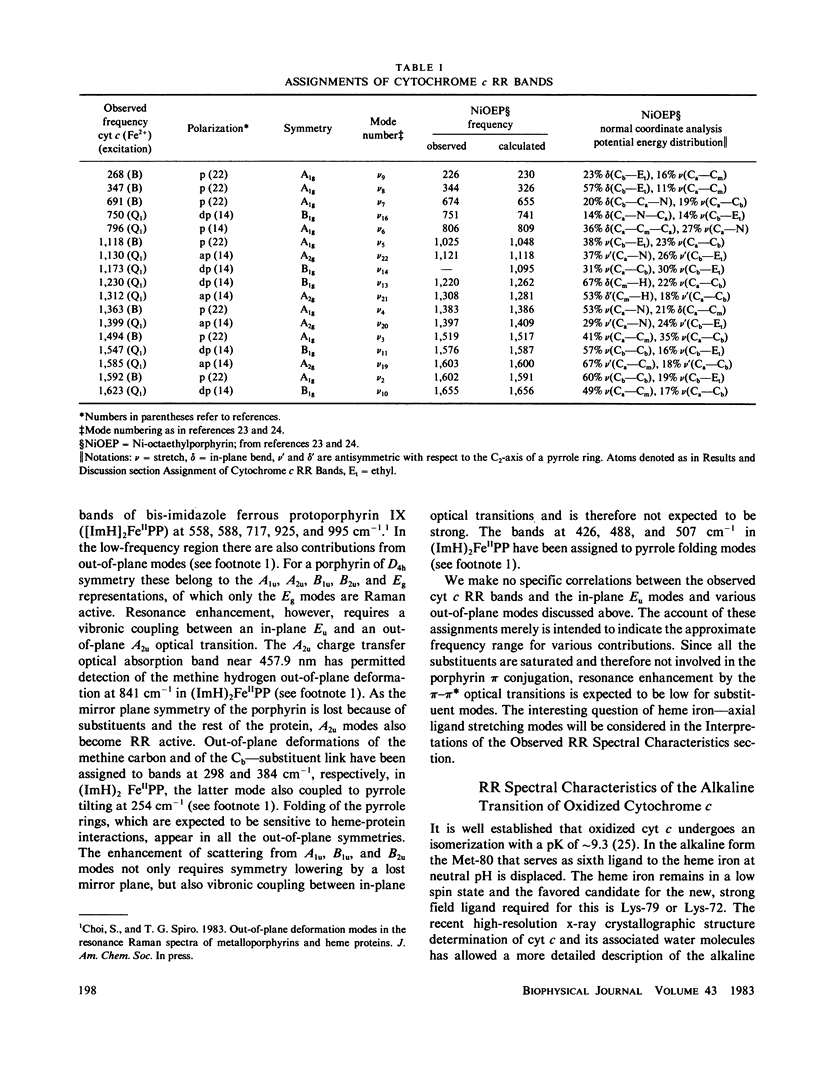
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenfeld L. A., Davydov R. M., Fel N. S., Magonov S. N., Vilu R. O. Studies on the conformational changes of metalloproteins induced by electrons in water-ethylene glycol solutions at low temperatures. Cytochrome C. FEBS Lett. 1974 Sep 1;45(1):256–258. doi: 10.1016/0014-5793(74)80856-2. [DOI] [PubMed] [Google Scholar]
- Blumenfeld L. A. The physical aspects of energy transduction in biological systems. Q Rev Biophys. 1978 Aug;11(3):251–308. doi: 10.1017/s0033583500002286. [DOI] [PubMed] [Google Scholar]
- Brautigan D. L., Feinberg B. A., Hoffman B. M., Margoliash E., Preisach J., Blumberg W. E. Multiple low spin forms of the cytochrome c ferrihemochrome. EPR spectra of various eukaryotic and prokaryotic cytochromes c. J Biol Chem. 1977 Jan 25;252(2):574–582. [PubMed] [Google Scholar]
- Cartling B., Ehrenberg A. A molecular mechanism of the energetic coupling of a sequence of electron transfer reactions to endergonic reactions. Biophys J. 1978 Sep;23(3):451–461. doi: 10.1016/S0006-3495(78)85461-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartling B., Wilbrandt R. Time-resolved resonance Raman spectroscopy of cytochrome c reduced by pulse radiolysis. Biochim Biophys Acta. 1981 Aug 12;637(1):61–68. doi: 10.1016/0005-2728(81)90210-3. [DOI] [PubMed] [Google Scholar]
- ESTABROOK R. W. The low temperature spectra of hemoproteins. I. Apparatus and its application to a study of cytochrome c. J Biol Chem. 1956 Dec;223(2):781–794. [PubMed] [Google Scholar]
- Eaton W. A., Hochstrasser R. M. Electronic spectrum of single crystals of ferricytochrome-c. J Chem Phys. 1967 Apr 1;46(7):2533–2539. doi: 10.1063/1.1841081. [DOI] [PubMed] [Google Scholar]
- Forster M., Hester R. E., Cartling B., Wilbrandt R. Continuous flow-resonance Raman spectroscopy of an intermediate redox state of cytochrome C. Biophys J. 1982 May;38(2):111–116. doi: 10.1016/S0006-3495(82)84537-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda-Saito M., Kitagawa T., Iizuka T., Kyogoku Y. Resonance Raman scattering from hemoproteins: pH-dependence of Raman spectra of ferrous dicarboxymethyl-methionyl-cytochrome c. FEBS Lett. 1975 Feb 1;50(2):233–235. doi: 10.1016/0014-5793(75)80495-9. [DOI] [PubMed] [Google Scholar]
- Kitagawa T., Kyogoku Y., Iizuka T., Ikeda-Saito M., Yamanaka T. Resonance Raman scattering from hemoproteins. Effects of ligands upon the Raman spectra of various C-type cytochromes. J Biochem. 1975 Oct;78(4):719–728. doi: 10.1093/oxfordjournals.jbchem.a130960. [DOI] [PubMed] [Google Scholar]
- Kitagawa T., Ozaki Y., Teraoka J., Kyogoku Y., Yamanaka T. The pH dependence of the resonance raman spectra and structural alterations at heme moieties of various c-type cytochromes. Biochim Biophys Acta. 1977 Sep 27;494(1):100–114. doi: 10.1016/0005-2795(77)90138-6. [DOI] [PubMed] [Google Scholar]
- Schejter A., Aviram I. The effects of alkylation of methionyl residues on the properties of horse cytochrome c. J Biol Chem. 1970 Apr 10;245(7):1552–1557. [PubMed] [Google Scholar]
- Simic M. G., Taub I. A. Mechanisms of inter- and intra-molecular electron transfer in cytochromes. Faraday Discuss Chem Soc. 1977;(63):270–278. doi: 10.1039/dc9776300270. [DOI] [PubMed] [Google Scholar]
- Spiro T. G., Burke J. M. Protein control of porphyrin conformation. Comparison of resonance Raman spectra of heme proteins with mesoporphyrin IX analogues. J Am Chem Soc. 1976 Sep 1;98(18):5482–5489. doi: 10.1021/ja00434a013. [DOI] [PubMed] [Google Scholar]
- Spiro T. G., Strekas T. C. Resonance Raman spectra of heme proteins. Effects of oxidation and spin state. J Am Chem Soc. 1974 Jan 23;96(2):338–345. doi: 10.1021/ja00809a004. [DOI] [PubMed] [Google Scholar]
- Spiro T. G., Strekas T. C. Resonance Raman spectra of hemoglobin and cytochrome c: inverse polarization and vibronic scattering. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2622–2626. doi: 10.1073/pnas.69.9.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T., Dickerson R. E. Conformation change of cytochrome c. I. Ferrocytochrome c structure refined at 1.5 A resolution. J Mol Biol. 1981 Nov 25;153(1):79–94. doi: 10.1016/0022-2836(81)90528-3. [DOI] [PubMed] [Google Scholar]
- Takano T., Dickerson R. E. Conformation change of cytochrome c. II. Ferricytochrome c refinement at 1.8 A and comparison with the ferrocytochrome structure. J Mol Biol. 1981 Nov 25;153(1):95–115. doi: 10.1016/0022-2836(81)90529-5. [DOI] [PubMed] [Google Scholar]
- Tasaki A., Otsuka J., Kotani M. Magnetic susceptibility measurements on hemoproteins down to 4.2 degrees K. Biochim Biophys Acta. 1967 Jun 27;140(2):284–290. doi: 10.1016/0005-2795(67)90469-2. [DOI] [PubMed] [Google Scholar]
- Van Wart H. E., Scheraga H. A. Raman and resonance raman spectroscopy. Methods Enzymol. 1978;49:67–149. doi: 10.1016/s0076-6879(78)49007-x. [DOI] [PubMed] [Google Scholar]
- van Leeuwen J. W., Tromp J., Nauta H. Reduction of ferricytochrome c, methemoglobin and metmyoglobin by hydroxyl and alcohol radicals. Biochim Biophys Acta. 1979 Apr 25;577(2):394–399. doi: 10.1016/0005-2795(79)90043-6. [DOI] [PubMed] [Google Scholar]


