Abstract
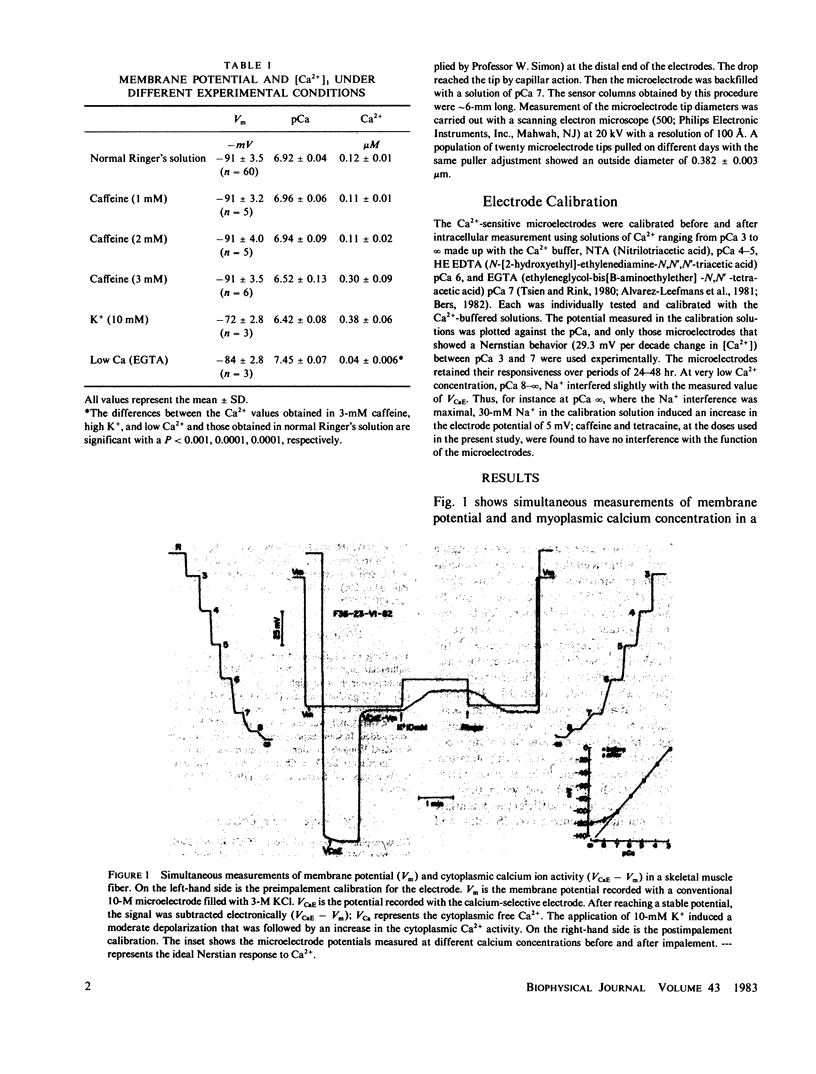
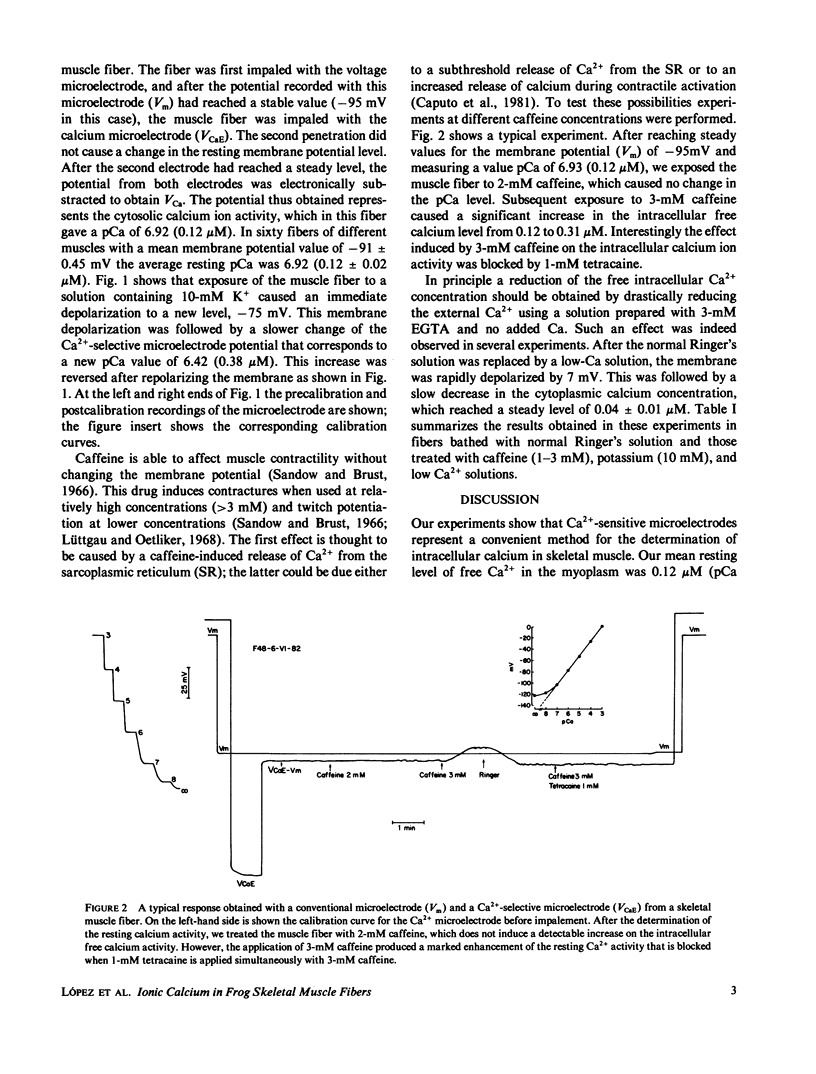
Ionic calcium concentrations were measured in frog skeletal muscle fibers using Ca-selective microelectrodes. In fibers with resting membrane potentials more negative than -85 mV, the mean pCa value was 6.94 (0.12 microM). In fibers depolarized to -73 mV with 10-mM K the mean pCa was 6.43 (0.37 microM). This increase in the intracellular [Ca2+] could be related to the higher oxygen consumption and heat production (Solandt effect) reported to occur under these conditions. Caffeine, 3 mM, also produced an increase in the free ionic calcium to a pCa of 6.52 (0.31 microM) without changes in the membrane potential. Lower caffeine concentrations, 1 and 2 mM, did not change the fiber pCa. Lower Ca concentrations in the external medium effectively reduced the internal ionic calcium to an estimated pCa of 7.43 (0.03 microM).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez-Leefmans F. J., Rink T. J., Tsien R. Y. Free calcium ions in neurones of Helix aspersa measured with ion-selective micro-electrodes. J Physiol. 1981 Jun;315:531–548. doi: 10.1113/jphysiol.1981.sp013762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo C., Gottschalk G., Lüttgau H. C. The control of contraction activation by the membrane potential. Experientia. 1981 Jun;37(6):580–581. doi: 10.1007/BF01990061. [DOI] [PubMed] [Google Scholar]
- DiPolo R., Rojas H., Vergara J., Lopez R., Caputo C. Measurements of intracellular ionized calcium in squid giant axons using calcium-selective electrodes. Biochim Biophys Acta. 1983 Mar 9;728(3):311–318. doi: 10.1016/0005-2736(83)90500-x. [DOI] [PubMed] [Google Scholar]
- Dipolo R., Requena J., Brinley F. J., Jr, Mullins L. J., Scarpa A., Tiffert T. Ionized calcium concentrations in squid axons. J Gen Physiol. 1976 Apr;67(4):433–467. doi: 10.1085/jgp.67.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttgau H. C., Oetliker H. The action of caffeine on the activation of the contractile mechanism in straited muscle fibres. J Physiol. 1968 Jan;194(1):51–74. doi: 10.1113/jphysiol.1968.sp008394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotný I., Vyskocil F. Possible role of Ca ions in the resting metabolism of frog sartorius muscle during potassium depolarization. J Cell Physiol. 1966 Feb;67(1):159–168. doi: 10.1002/jcp.1040670118. [DOI] [PubMed] [Google Scholar]
- Solandt D. Y. The effect of potassium on the excitability and resting metabolism of frog's muscle. J Physiol. 1936 Feb 8;86(2):162–170. doi: 10.1113/jphysiol.1936.sp003351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J. Neutral carrier ion-selective microelectrodes for measurement of intracellular free calcium. Biochim Biophys Acta. 1980 Jul;599(2):623–638. doi: 10.1016/0005-2736(80)90205-9. [DOI] [PubMed] [Google Scholar]



