Abstract
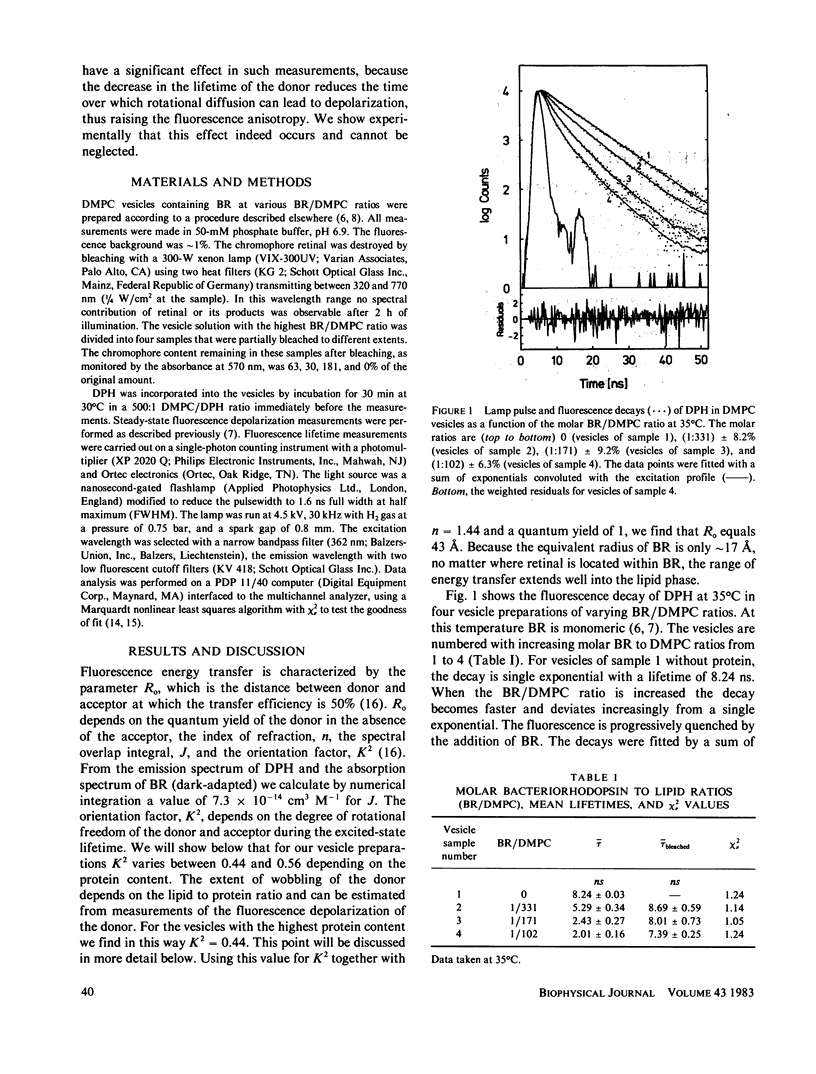
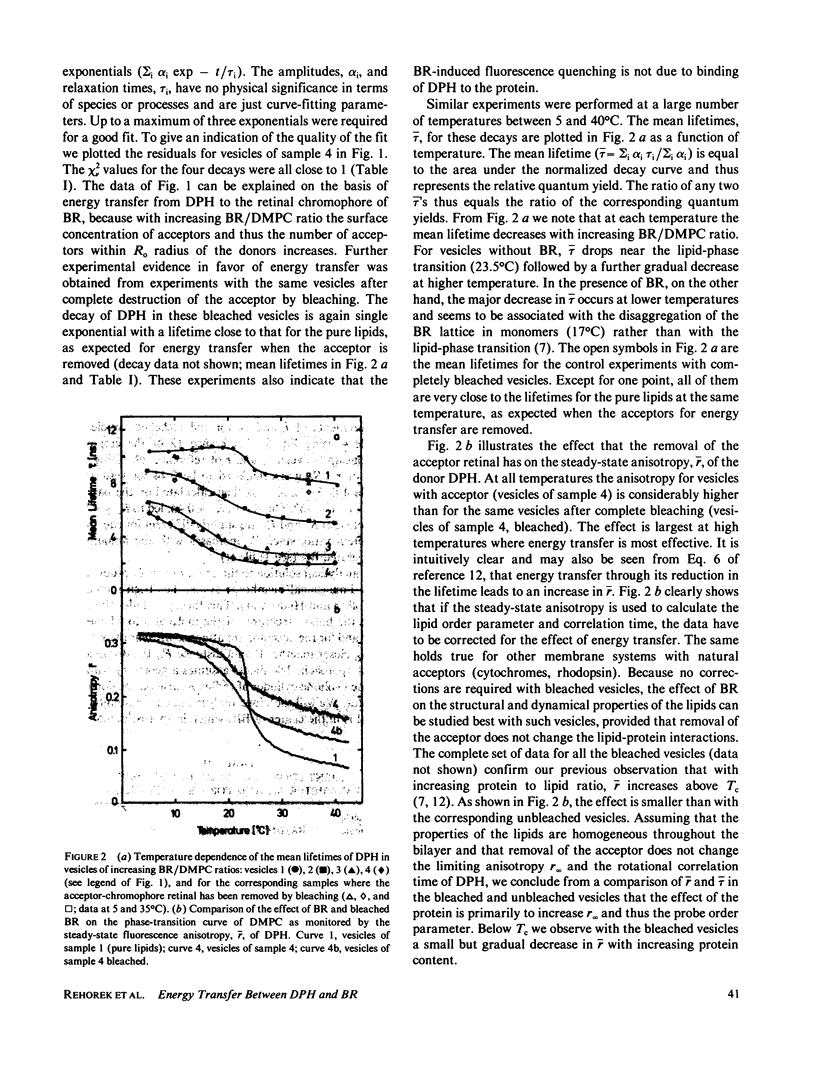
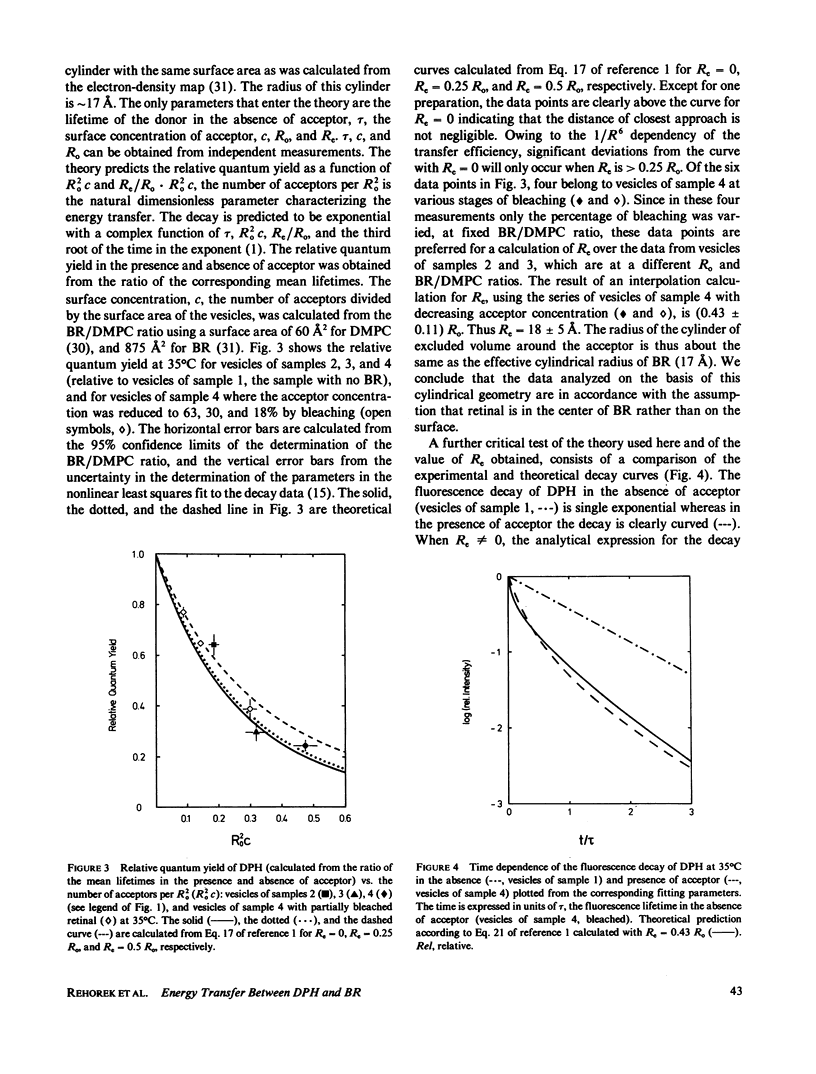
Fluorescence energy transfer between the donor diphenylhexatriene (DPH) and the acceptor retinal and fluorescence depolarization of DPH are used to test current theories for fluorescence energy transfer in two-dimensional systems and to obtain information on the effect of the intrinsic membrane protein, bacteriorhodopsin, on the order and dynamics of the lipid phase. Increasing the surface concentration of acceptors by raising the protein to lipid ratio leads to a decrease in the mean fluorescence lifetime by up to a factor of four. When the acceptor concentration is reduced at a fixed protein to lipid ratio by photochemical destruction of retinal, the lifetime increases and reaches approximately the value observed in protein-free vesicles when the bleaching is complete. The shape of the decay curve and the dependency of the mean lifetime on the surface concentration of acceptors are in agreement with theoretical predictions for a two-dimensional random distribution of donors and acceptors. From this analysis a distance of closest approach between donors and acceptors of approximately 18 A is obtained, which is close to the effective radius of bacteriorhodopsin (17 A) and consistent with current ideas about the location of retinal in the interior of the protein. In the absence of energy transfer (bleached vesicles), the steady-state fluorescence anisotropy, -r, of DPH is considerably lower than in the corresponding unbleached vesicles, indicating that the effect of energy transfer must be taken into account when interpreting -r in terms of order and dynamics.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrich M. P., Vanderkooi J. M. Temperature dependence of 1,6-diphenyl-1,3,5-hexatriene fluorescence in phophoslipid artificial membranes. Biochemistry. 1976 Mar 23;15(6):1257–1261. doi: 10.1021/bi00651a013. [DOI] [PubMed] [Google Scholar]
- Bayley H., Huang K. S., Radhakrishnan R., Ross A. H., Takagaki Y., Khorana H. G. Site of attachment of retinal in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2225–2229. doi: 10.1073/pnas.78.4.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. A., Dale R. E., Roth S., Brand L. Nanosecond time-dependent fluorescence depolarization of diphenylhexatriene in dimyristoyllecithin vesicles and the determination of "microviscosity". J Biol Chem. 1977 Apr 10;252(7):2163–2169. [PubMed] [Google Scholar]
- Cherry R. J., Müller U. Temperature-dependent aggregation of bacteriorhodopsin in dipalmitoyl- and dimyristoylphosphatidylcholine vesicles. J Mol Biol. 1978 May 15;121(2):283–298. doi: 10.1016/s0022-2836(78)80010-2. [DOI] [PubMed] [Google Scholar]
- Curatolo W., Sakura J. D., Small D. M., Shipley G. G. Protein-lipid interactions: recombinants of the proteolipid apoprotein of myelin with dimyristoyllecithin. Biochemistry. 1977 May 31;16(11):2313–2319. doi: 10.1021/bi00630a001. [DOI] [PubMed] [Google Scholar]
- Dale R. E., Eisinger J., Blumberg W. E. The orientational freedom of molecular probes. The orientation factor in intramolecular energy transfer. Biophys J. 1979 May;26(2):161–193. doi: 10.1016/S0006-3495(79)85243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dencher N. A., Heyn M. P. Bacteriorhodopsin monomers pump protons. FEBS Lett. 1979 Dec 15;108(2):307–310. doi: 10.1016/0014-5793(79)80552-9. [DOI] [PubMed] [Google Scholar]
- Dewey T. G., Hammes G. G. Calculation on fluorescence resonance energy transfer on surfaces. Biophys J. 1980 Dec;32(3):1023–1035. doi: 10.1016/S0006-3495(80)85033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estep T. N., Thompson T. E. Energy transfer in lipid bilayers. Biophys J. 1979 May;26(2):195–207. doi: 10.1016/S0006-3495(79)85244-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung B. K., Stryer L. Surface density determination in membranes by fluorescence energy transfer. Biochemistry. 1978 Nov 28;17(24):5241–5248. doi: 10.1021/bi00617a025. [DOI] [PubMed] [Google Scholar]
- Grinvald A. The use of standards in the analysis of fluorescence decay data. Anal Biochem. 1976 Sep;75(1):260–280. doi: 10.1016/0003-2697(76)90077-4. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Heyn M. P., Blume A., Rehorek M., Dencher N. A. Calorimetric and fluorescence depolarization studies on the lipid phase transition of bacteriorhodopsin--dimyristoylphosphatidylcholine vesicles. Biochemistry. 1981 Dec 8;20(25):7109–7115. doi: 10.1021/bi00528a009. [DOI] [PubMed] [Google Scholar]
- Heyn M. P., Cherry R. J., Dencher N. A. Lipid--protein interactions in bacteriorhodopsin--dimyristoylphosphatidylcholine vesicles. Biochemistry. 1981 Feb 17;20(4):840–849. doi: 10.1021/bi00507a029. [DOI] [PubMed] [Google Scholar]
- Heyn M. P., Cherry R. J., Müller U. Transient and linear dichroism studies on bacteriorhodopsin: determination of the orientation of the 568 nm all-trans retinal chromophore. J Mol Biol. 1977 Dec 15;117(3):607–620. doi: 10.1016/0022-2836(77)90060-2. [DOI] [PubMed] [Google Scholar]
- Heyn M. P. Determination of lipid order parameters and rotational correlation times from fluorescence depolarization experiments. FEBS Lett. 1979 Dec 15;108(2):359–364. doi: 10.1016/0014-5793(79)80564-5. [DOI] [PubMed] [Google Scholar]
- Jähnig F. Structural order of lipids and proteins in membranes: evaluation of fluorescence anisotropy data. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6361–6365. doi: 10.1073/pnas.76.12.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawato S., Kinosita K., Jr, Ikegami A. Dynamic structure of lipid bilayers studied by nanosecond fluorescence techniques. Biochemistry. 1977 May 31;16(11):2319–2324. doi: 10.1021/bi00630a002. [DOI] [PubMed] [Google Scholar]
- King G. I., Mowery P. C., Stoeckenius W., Crespi H. L., Schoenborn B. P. Location of the chromophore in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4726–4730. doi: 10.1073/pnas.77.8.4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. I., Stoekenius W., Crespi H. L., Schoenborn B. P. The location of retinal in the purple membrane profile by neutron diffraction. J Mol Biol. 1979 Jun 5;130(4):395–404. doi: 10.1016/0022-2836(79)90430-3. [DOI] [PubMed] [Google Scholar]
- Kinosita K., Jr, Kataoka R., Kimura Y., Gotoh O., Ikegami A. Dynamic structure of biological membranes as probed by 1,6-diphenyl-1,3,5-hexatriene: a nanosecond fluorescence depolarization study. Biochemistry. 1981 Jul 21;20(15):4270–4277. doi: 10.1021/bi00518a006. [DOI] [PubMed] [Google Scholar]
- Kinosita K., Jr, Kawato S., Ikegami A. A theory of fluorescence polarization decay in membranes. Biophys J. 1977 Dec;20(3):289–305. doi: 10.1016/S0006-3495(77)85550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinosita K., Jr, Kawato S., Ikegami A., Yoshida S., Orii Y. The effect of cytochrome oxidase on lipid chain dynamics. A nanosecond fluorescence depolarization study. Biochim Biophys Acta. 1981 Sep 21;647(1):7–17. doi: 10.1016/0005-2736(81)90290-x. [DOI] [PubMed] [Google Scholar]
- Koppel D. E., Fleming P. J., Strittmatter P. Intramembrane positions of membrane-bound chromophores determined by excitation energy transfer. Biochemistry. 1979 Nov 27;18(24):5450–5457. doi: 10.1021/bi00591a030. [DOI] [PubMed] [Google Scholar]
- Kouyama T., Kimura Y., Kinosita K., Jr, Ikegami A. Location and orientation of the chromophore in bacteriorhodopsin. Analysis by fluorescence energy transfer. J Mol Biol. 1981 Dec 5;153(2):337–359. doi: 10.1016/0022-2836(81)90282-5. [DOI] [PubMed] [Google Scholar]
- Lakowicz J. R., Prendergast F. G., Hogen D. Differential polarized phase fluorometric investigations of diphenylhexatriene in lipid bilayers. Quantitation of hindered depolarizing rotations. Biochemistry. 1979 Feb 6;18(3):508–519. doi: 10.1021/bi00570a021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman W. V., Caplan S. R. Influence of membrane lipids on the photochemistry of bacteriorhodopsin in the purple membrane of Halobacterium halobium. Biochim Biophys Acta. 1978 May 10;502(2):222–231. doi: 10.1016/0005-2728(78)90044-0. [DOI] [PubMed] [Google Scholar]
- Stryer L. Fluorescence energy transfer as a spectroscopic ruler. Annu Rev Biochem. 1978;47:819–846. doi: 10.1146/annurev.bi.47.070178.004131. [DOI] [PubMed] [Google Scholar]
- Van Blitterswijk W. J., Van Hoeven R. P., Van der Meer B. W. Lipid structural order parameters (reciprocal of fluidity) in biomembranes derived from steady-state fluorescence polarization measurements. Biochim Biophys Acta. 1981 Jun 22;644(2):323–332. doi: 10.1016/0005-2736(81)90390-4. [DOI] [PubMed] [Google Scholar]
- Wolber P. K., Hudson B. S. An analytic solution to the Förster energy transfer problem in two dimensions. Biophys J. 1979 Nov;28(2):197–210. doi: 10.1016/S0006-3495(79)85171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


