Abstract
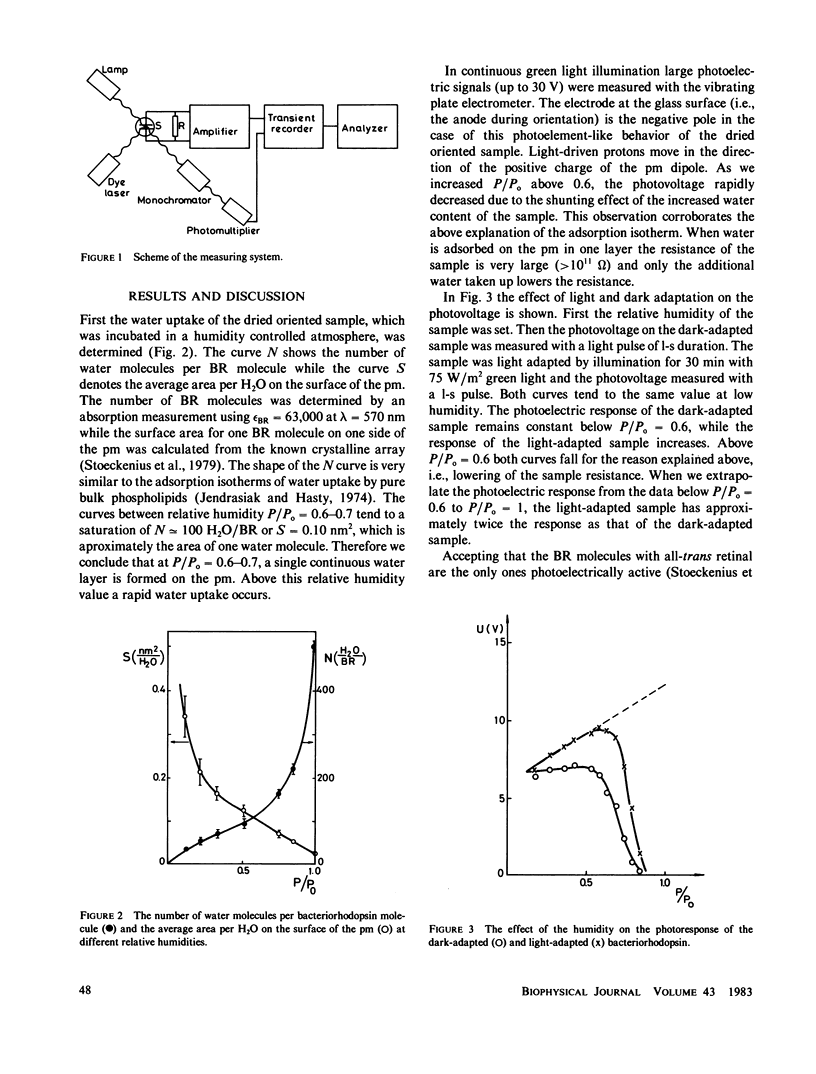
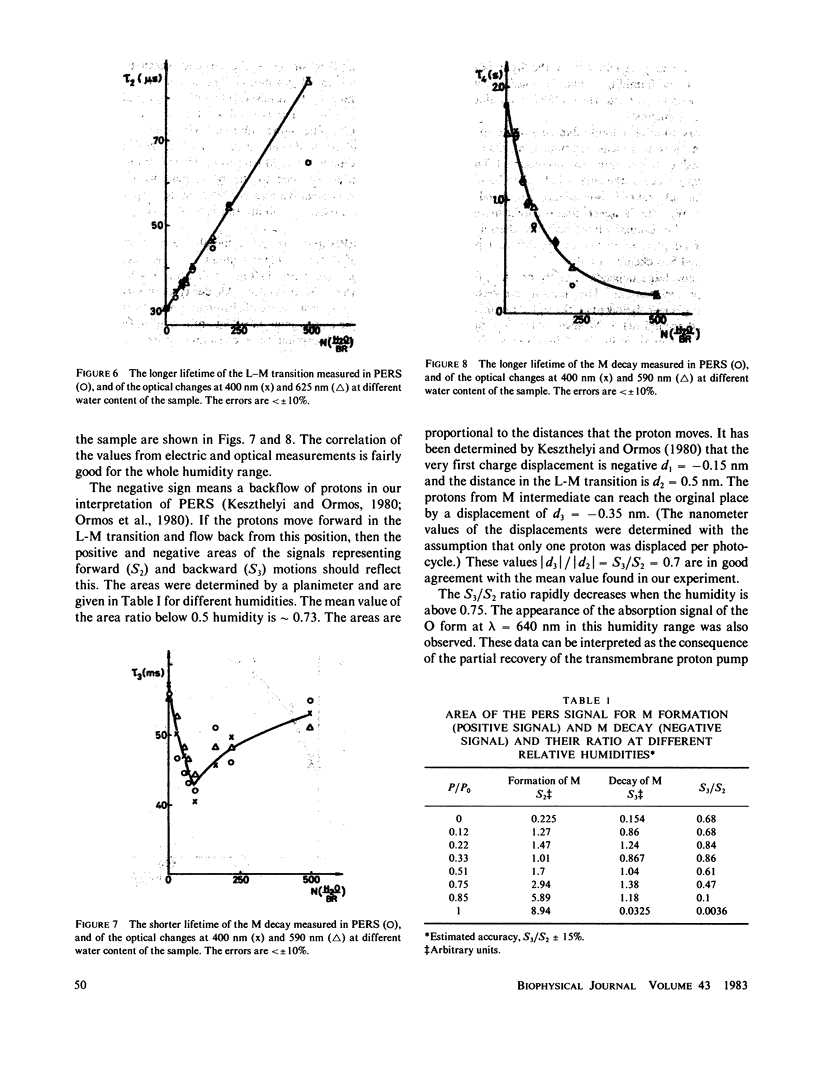
In dried oriented samples of purple membrane isolated from Halobacterium halobium, the photoelectric activity decreases and the light adaptation vanishes when the water content of the sample is lowered. In the photocycle the first steps of the proton movement were accelerated with decreasing humidity, while the last steps of the photocycle could not be observed. From the analysis of the photoelectric signal we conclude that at low humidities the protons move forward in the L decay and return to their original place during M decay.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin R. H., Beeson K. W., Eisenstein L., Frauenfelder H., Gunsalus I. C. Dynamics of ligand binding to myoglobin. Biochemistry. 1975 Dec 2;14(24):5355–5373. doi: 10.1021/bi00695a021. [DOI] [PubMed] [Google Scholar]
- Barabás K., Dér A., Dancsházy Z., Ormos P., Keszthelyi L., Marden M. Electro-optical measurements on aqueous suspension of purple membrane from Halobacterium halobium. Biophys J. 1983 Jul;43(1):5–11. doi: 10.1016/S0006-3495(83)84317-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadio R., Stoeckenius W. Effect of protein-protein interaction on light adaptation of bacteriorhodopsin. Biochemistry. 1980 Jul 8;19(14):3374–3381. doi: 10.1021/bi00555a043. [DOI] [PubMed] [Google Scholar]
- Fisher K. A., Yanagimoto K., Stoeckenius W. Oriented adsorption of purple membrane to cationic surfaces. J Cell Biol. 1978 May;77(2):611–621. doi: 10.1083/jcb.77.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jendrasiak G. L., Hasty J. H. The hydration of phospholipids. Biochim Biophys Acta. 1974 Jan 23;337(1):79–91. doi: 10.1016/0005-2760(74)90042-3. [DOI] [PubMed] [Google Scholar]
- Kalisky O., Ottolenghi M., Honig B., Korenstein R. Environmental effects on formation and photoreaction of the M412 photoproduct of bacteriorhodopsin: implications for the mechanism of proton pumping. Biochemistry. 1981 Feb 3;20(3):649–655. doi: 10.1021/bi00506a031. [DOI] [PubMed] [Google Scholar]
- Korenstein R., Hess B. Hydration effects on cis--trans isomerization of bacteriorhodopsin. FEBS Lett. 1977 Oct 1;82(1):7–11. doi: 10.1016/0014-5793(77)80874-0. [DOI] [PubMed] [Google Scholar]
- Korenstein R., Hess B. Hydration effects on the photocycle of bacteriorhodopsin in thin layers of purple membrane. Nature. 1977 Nov 10;270(5633):184–186. doi: 10.1038/270184a0. [DOI] [PubMed] [Google Scholar]
- Nagy K. Photoelectric activity of dried, oriented layers of purple membrane from Halobacterium halobium. Biochem Biophys Res Commun. 1978 Nov 14;85(1):383–390. doi: 10.1016/s0006-291x(78)80054-0. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Ormos P., Dancsházy Z., Keszthelyi L. Electric response of a back photoreaction in the bacteriorhodopsin photocycle. Biophys J. 1980 Aug;31(2):207–213. doi: 10.1016/S0006-3495(80)85051-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckenius W., Lozier R. H., Bogomolni R. A. Bacteriorhodopsin and the purple membrane of halobacteria. Biochim Biophys Acta. 1979 Mar 14;505(3-4):215–278. doi: 10.1016/0304-4173(79)90006-5. [DOI] [PubMed] [Google Scholar]


