Abstract
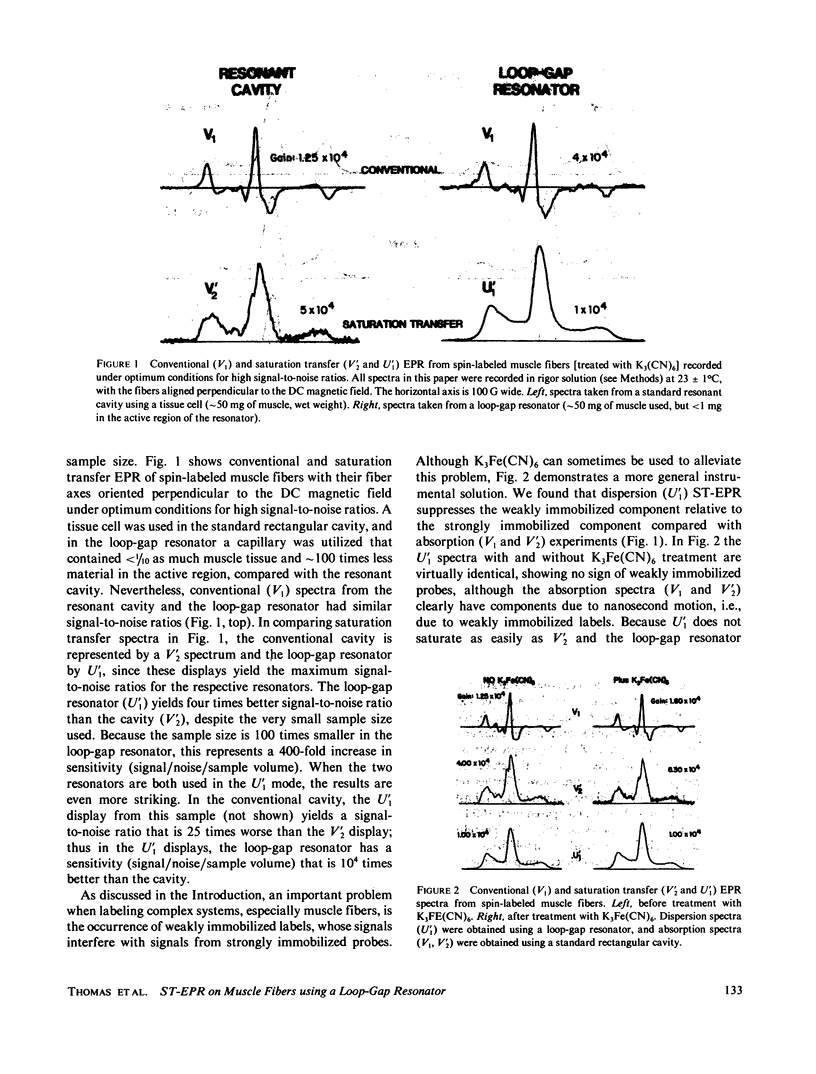
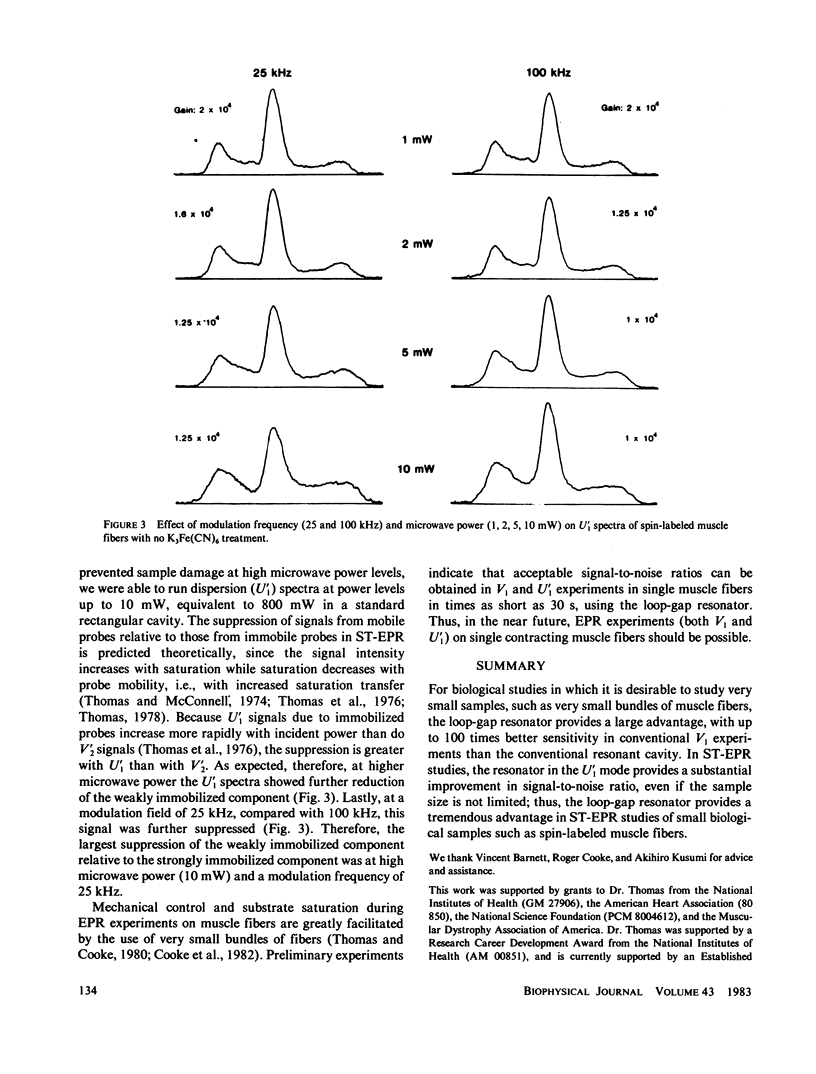
Previously, saturation transfer (ST-EPR) studies of biomolecular dynamics have involved the use of a resonant cavity and the V'2 display (absorption, second harmonic, out of phase). In the present study, we replaced the resonant cavity with a loop-gap resonator and used the U'1 display (dispersion, first harmonic, out of phase) to study spin-labeled muscle fibers. The new resonator and display showed several advantages over those previously used. It produced virtually noiseless U'1 spectra on a 0.4 microliter sample using a 4 min scan; previous U'1 experiments on spin-labeled muscle, using a conventional rectangular cavity, resulted in an unacceptably low signal-to-noise ratio. The high filling factor of the resonator facilitated the study of these extremely small fiber bundles and permitted high microwave field intensities to be achieved at much lower incident microwave power levels, thus greatly enhancing the signal-to-noise ratio in U'1 experiments. This reduction in the noise level made it possible to benefit from the other advantages of U'1 over V'2, such as stronger signals, simpler line shapes, and simpler data analysis. For these muscle fiber samples, the resulting sensitivity (signal/noise/sample volume) of the U'1 signals was greater than 100 times that of V'2 signals obtained in a conventional cavity. Another advantage of the U'1 display is that signals from weakly immobilized probes, i.e., probes that have nanosecond rotational mobility relative to the labeled protein (myosin), are greatly suppressed relative to strongly immobilized probes. This reduces the ambiguity of spectral analysis, and eliminates the need for chemical treatments [e.g., using K3Fe(CN)6] that were previously required in muscle fibers and other systems. Further suppression of this weakly immobilized component was achieved in U'1 spectra by increasing the microwave power and decreasing the field modulation frequency.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooke R., Crowder M. S., Thomas D. D. Orientation of spin labels attached to cross-bridges in contracting muscle fibres. Nature. 1982 Dec 23;300(5894):776–778. doi: 10.1038/300776a0. [DOI] [PubMed] [Google Scholar]
- Graceffa P., Seidel J. C. A reaction involving protein sulfhydryl groups, a bound spin-label, and K3Fe(CN)6 as a probe of sulfhydryl proximity in myosin. Biochemistry. 1980 Jan 8;19(1):33–39. doi: 10.1021/bi00542a006. [DOI] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Seidel J. C., Chopek M., Gergely J. Effect of nucleotides and pyrophosphate on spin labels bound to S1 thiol groups of myosin. Biochemistry. 1970 Aug 4;9(16):3265–3272. doi: 10.1021/bi00818a021. [DOI] [PubMed] [Google Scholar]
- Thomas D. D., Cooke R. Orientation of spin-labeled myosin heads in glycerinated muscle fibers. Biophys J. 1980 Dec;32(3):891–906. doi: 10.1016/S0006-3495(80)85024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Ishiwata S., Seidel J. C., Gergely J. Submillisecond rotational dynamics of spin-labeled myosin heads in myofibrils. Biophys J. 1980 Dec;32(3):873–889. doi: 10.1016/S0006-3495(80)85023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D. Large-scale rotational motions of proteins detected by electron paramagnetic resonance and fluorescence. Biophys J. 1978 Nov;24(2):439–462. doi: 10.1016/S0006-3495(78)85394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Seidel J. C., Hyde J. S., Gergely J. Motion of subfragment-1 in myosin and its supramolecular complexes: saturation transfer electron paramagnetic resonance. Proc Natl Acad Sci U S A. 1975 May;72(5):1729–1733. doi: 10.1073/pnas.72.5.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregear R. T., Marston S. B. The crossbridge theory. Annu Rev Physiol. 1979;41:723–736. doi: 10.1146/annurev.ph.41.030179.003451. [DOI] [PubMed] [Google Scholar]


