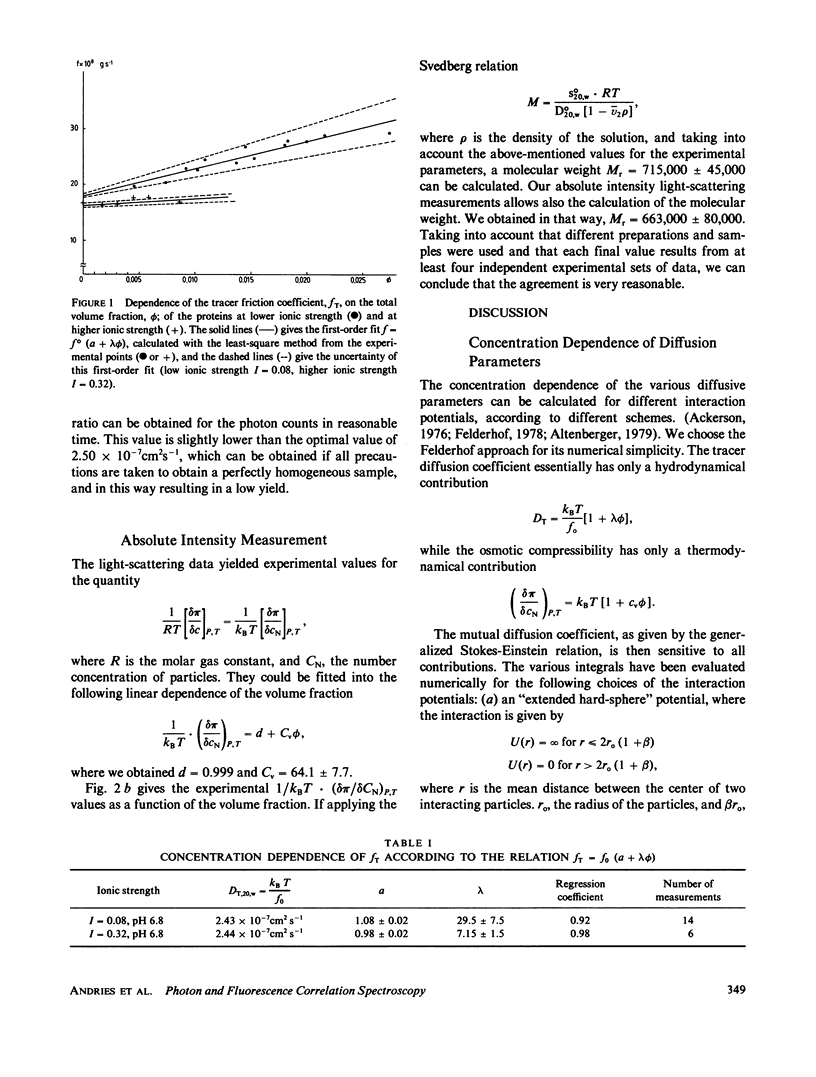
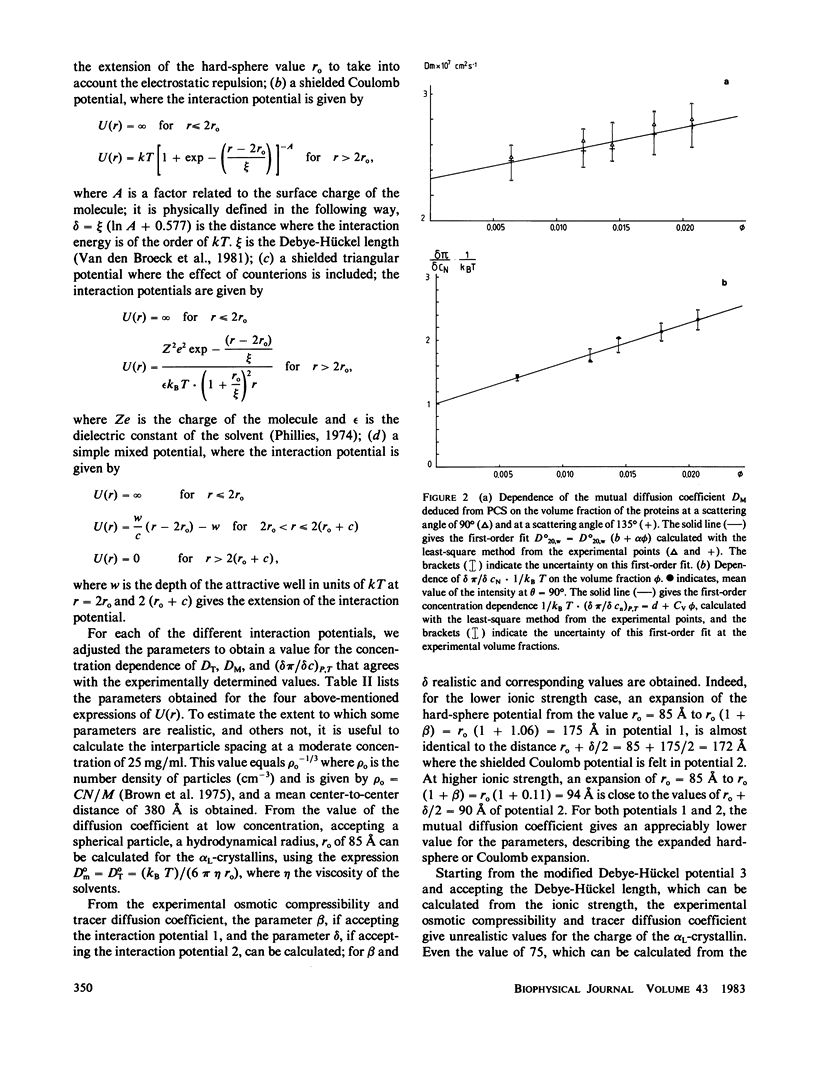
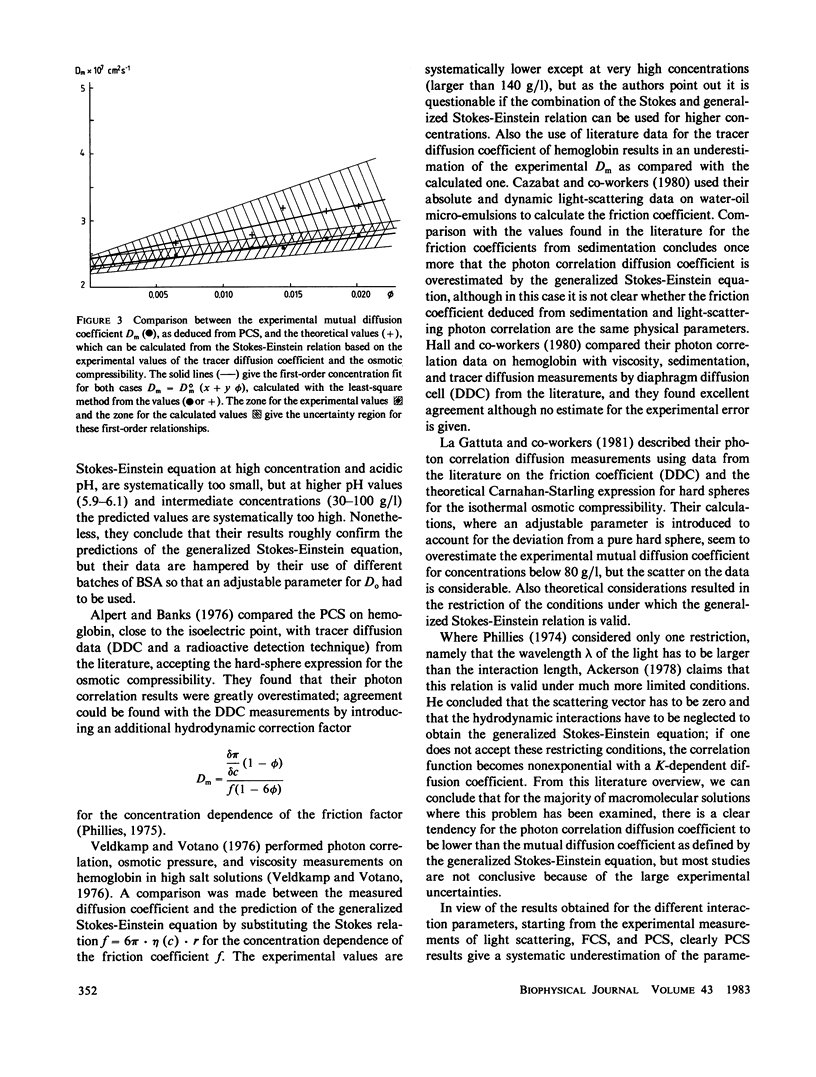
Abstract
The bovine eye-lens protein, alpha L-crystallin, has been studied with photon correlation spectroscopy to obtain the mutual diffusion coefficient, Dm, with fluorescence correlation spectroscopy to determine the tracer diffusion coefficient, DT, and with light scattering to get the isothermal osmotic compressibility (delta pi/delta c) P,T. The concentration dependence of Dm, DT, and (delta pi/delta c) P,T up to a volume fraction phi of the protein of 2.5 x 10(-2) has been interpreted on the basis of four different interaction potentials: (a) an extended hard-sphere potential; (b) a shielded Coulomb potential; (c) a shielded Coulomb interaction where the effect of counterions is included; (d) a simple mixed potential. The three parameters Dm, DT, and (delta pi/delta c) P,T have also been combined in the generalized Stokes-Einstein equation, Dm = [(delta pi/delta c)P,T . (1--phi) . (DT)]/(kappa B . T). Our results indicate that, in the case that photon correlation spectroscopy gives the mutual diffusion coefficient Dm, the applicability of the Stokes-Einstein equation can be questioned; or that, when one assumes the Stokes-Einstein equation to be valid, there is significant discrepancy between the result of photon correlation spectroscopy and Dm.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpert S. S., Banks G. The concentration dependence of the hemoglobin mutual diffusion coefficient. Biophys Chem. 1976 May;4(3):287–296. doi: 10.1016/0301-4622(76)80077-4. [DOI] [PubMed] [Google Scholar]
- Andries C., Backhovens H., Clauwaert J., De Block J., De Voeght F., Dhont C. Physical-chemical studies on bovine eye lens proteins. I. Light-scattering and viscosity studies of low-molecular weight alpha-crystallin isolated from adult and embryonic bovine lenses. Exp Eye Res. 1982 Feb;34(2):239–255. doi: 10.1016/0014-4835(82)90058-6. [DOI] [PubMed] [Google Scholar]
- Bettelheim F. A., Bettelheim A. A. Small-angle light scattering studies on xylose cataract formation in bovine lenses. Invest Ophthalmol Vis Sci. 1978 Sep;17(9):896–902. [PubMed] [Google Scholar]
- Bettelheim F. A. On the optical anisotropy of lens fiber cells. Exp Eye Res. 1975 Sep;21(3):231–234. doi: 10.1016/0014-4835(75)90093-7. [DOI] [PubMed] [Google Scholar]
- Bettelheim F. A., Paunovic M. Light scattering of normal human lens I. Application of random density and orientation fluctuation theory. Biophys J. 1979 Apr;26(1):85–99. doi: 10.1016/S0006-3495(79)85237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemendal H. Lens proteins. CRC Crit Rev Biochem. 1982;12(1):1–38. doi: 10.3109/10409238209105849. [DOI] [PubMed] [Google Scholar]
- Bloomfield V. A., Lim T. K. Quasi-elastic laser light scattering. Methods Enzymol. 1978;48:415–494. doi: 10.1016/s0076-6879(78)48021-8. [DOI] [PubMed] [Google Scholar]
- Delaye M., Clark J. I., Benedek G. B. Identification of the scattering elements responsible for lens opacification in cold cataracts. Biophys J. 1982 Mar;37(3):647–656. [PMC free article] [PubMed] [Google Scholar]
- Duncan G., Bushell A. R. Ion analyses of human cataractous lenses. Exp Eye Res. 1975 Mar;20(3):223–230. doi: 10.1016/0014-4835(75)90136-0. [DOI] [PubMed] [Google Scholar]
- GOSTING L. J. Measurement and interpretation of diffusion coefficients of proteins. Adv Protein Chem. 1956;11:429–554. doi: 10.1016/s0065-3233(08)60425-8. [DOI] [PubMed] [Google Scholar]
- Harding J. J., Dilley K. J. Structural proteins of the mammalian lens: a review with emphasis on changes in development, aging and cataract. Exp Eye Res. 1976 Jan;22(1):1–73. doi: 10.1016/0014-4835(76)90033-6. [DOI] [PubMed] [Google Scholar]
- Ishimoto C., Goalwin P. W., Sun S. T., Nishio I., Tanaka T. Cytoplasmic phase separation in formation of galactosemic cataract in lenses of young rats. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4414–4416. doi: 10.1073/pnas.76.9.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel D. E., Axelrod D., Schlessinger J., Elson E. L., Webb W. W. Dynamics of fluorescence marker concentration as a probe of mobility. Biophys J. 1976 Nov;16(11):1315–1329. doi: 10.1016/S0006-3495(76)85776-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaGattuta K. J., Sharma V. S., Nicoli D. F., Kothari B. K. Diffusion coefficients of hemoglobin by intensity fluctuation spectroscopy: effects of varying pH and ionic strength. Biophys J. 1981 Jan;33(1):63–79. doi: 10.1016/S0006-3495(81)84872-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Benedek G. B. Observation of protein diffusivity in intact human and bovine lenses with application to cataract. Invest Ophthalmol. 1975 Jun;14(6):449–456. [PubMed] [Google Scholar]
- Tanaka T., Ishimoto C., Chylack L. T., Jr Phase separation of a protein-water mixture in cold cataract in the young rat lens. Science. 1977 Sep 2;197(4307):1010–1012. doi: 10.1126/science.887936. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Ishimoto C. In vivo observation of protein diffusivity in rabbit lenses. Invest Ophthalmol Vis Sci. 1977 Feb;16(2):135–140. [PubMed] [Google Scholar]


