Abstract
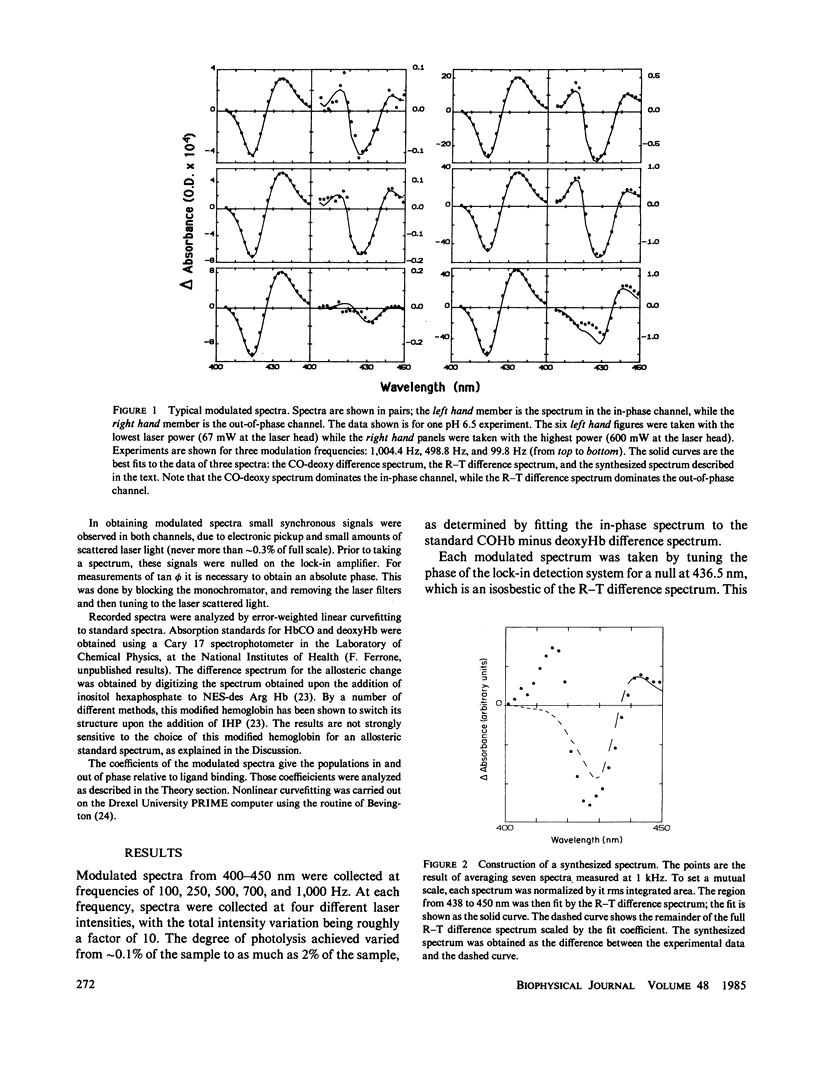
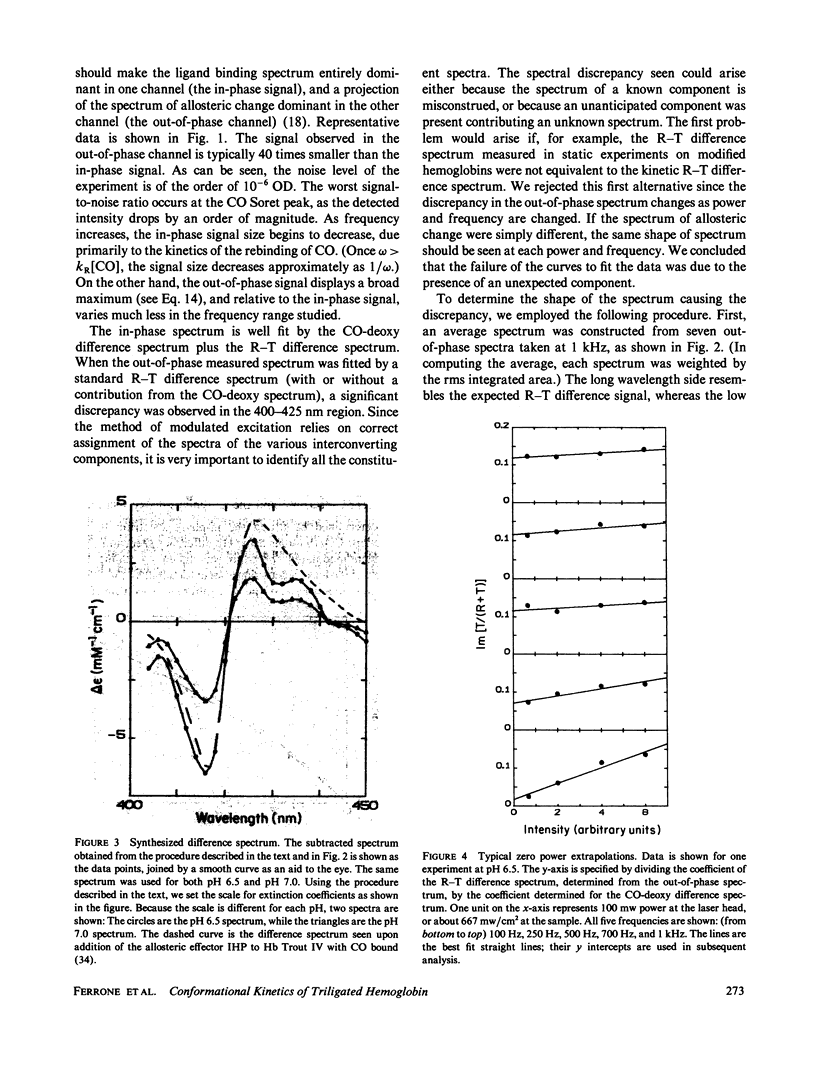
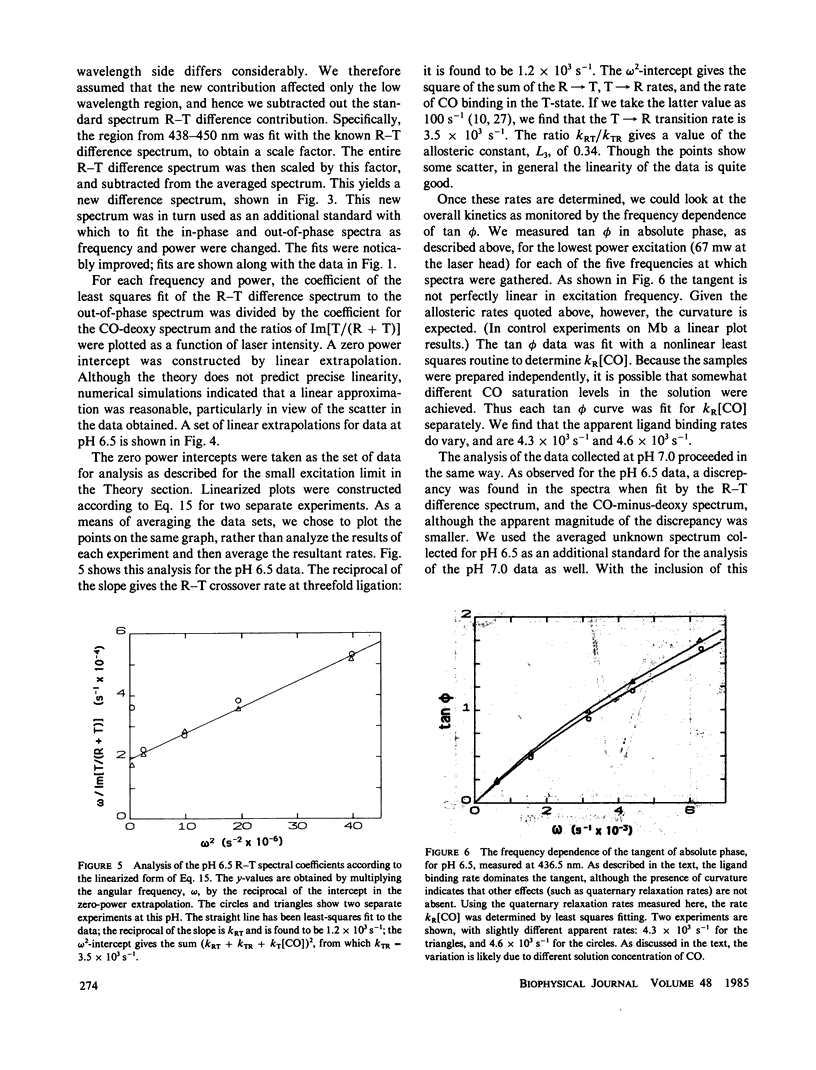
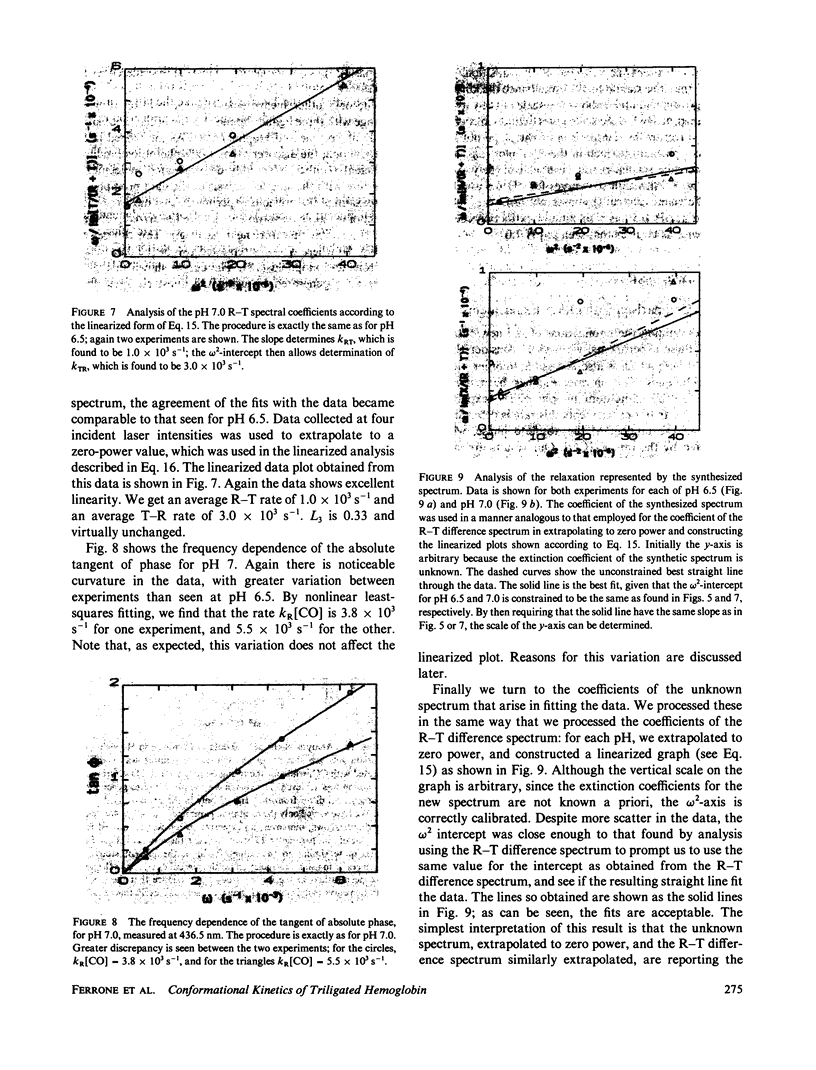
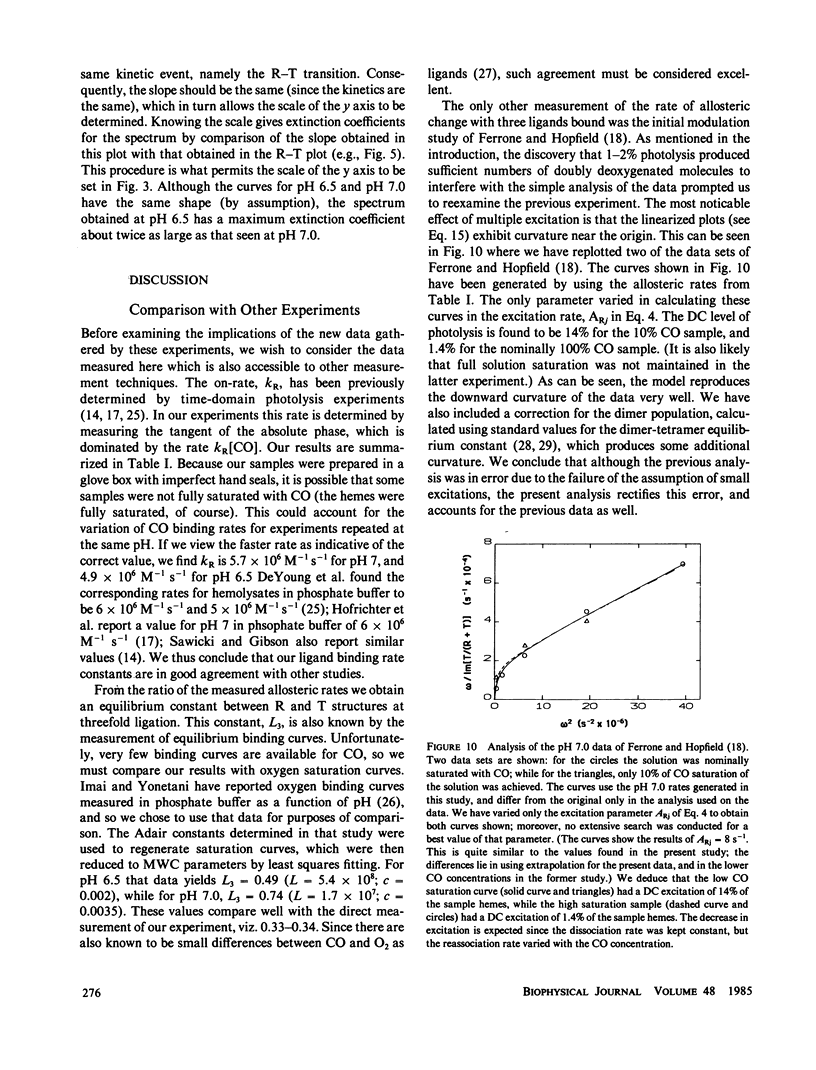
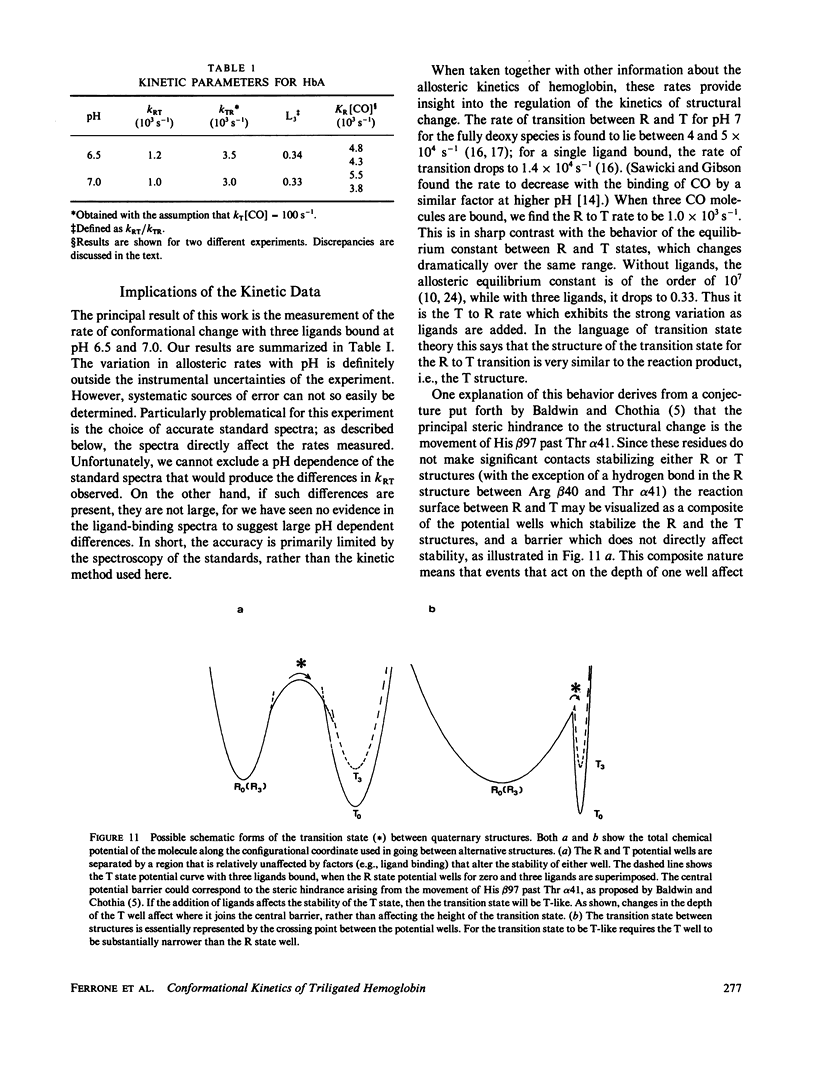
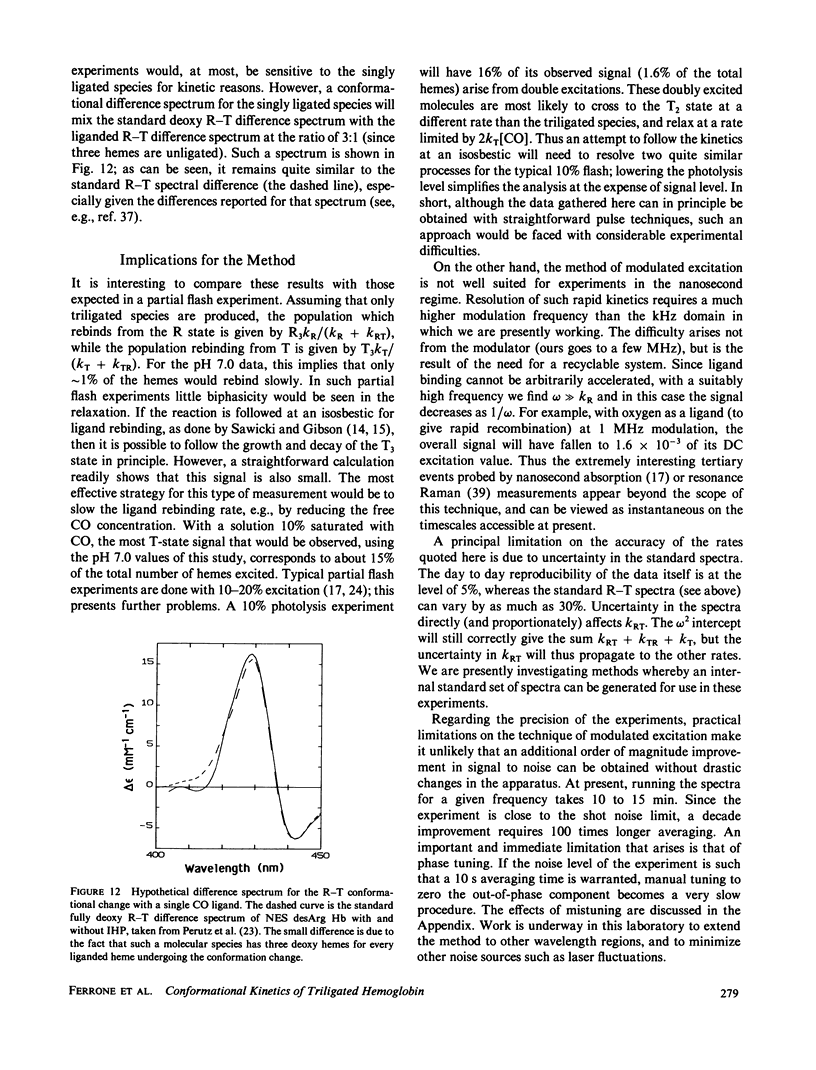
We have used the method of modulated excitation (Ferrone, F.A., and J.J. Hopfield, 1976, Proc. Natl. Acad. Sci. USA. 73:4497-4501), with an improved apparatus and a revised analytical procedure, to measure the rate of conformational change between the oxy (R) and deoxy (T) conformations of triligated carboxy-hemoglobin A at pH 6.5 and 7.0. We have found the rates to be kRT = 1.2 X 10(3) s-1 and kTR = 3.5 X 10(3) s-1 for pH 6.5, while for pH 7.0, kRT = 1.0 X 10(3) s-1, and kTR = 3.0 X 10(3) s-1. The value for L3, the equilibrium constant between conformations, was virtually unchanged between pH 6.5 and 7.0. While the rates measured here differ from those obtained in the original use of this method, these new rates are fully consistent with the original data when analyzed by the revised procedures presented here. When taken with other kinetic and equilibrium data, our measurements suggest that the transition state between structures is dominated by the behavior of the T quaternary structure. Finally, a spectral feature near the HbCO Soret peak has been observed that we ascribe to an allosteric perturbation of the spectra of the liganded hemes.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin J., Chothia C. Haemoglobin: the structural changes related to ligand binding and its allosteric mechanism. J Mol Biol. 1979 Apr 5;129(2):175–220. doi: 10.1016/0022-2836(79)90277-8. [DOI] [PubMed] [Google Scholar]
- Careri G., Fasella P., Gratton E. Enzyme dynamics: the statistical physics approach. Annu Rev Biophys Bioeng. 1979;8:69–97. doi: 10.1146/annurev.bb.08.060179.000441. [DOI] [PubMed] [Google Scholar]
- Castillo C. L., Ogawa S., Salhany J. M. Equilibrium and kinetic measurements of carbon monoxide binding to hemoglobin Kansas in the presence of inositol hexaphosphate. Arch Biochem Biophys. 1978 Jan 30;185(2):504–510. doi: 10.1016/0003-9861(78)90195-9. [DOI] [PubMed] [Google Scholar]
- Cho K. C., Hopfield J. J. Spin equilibrium and quaternary structure change in hemoglobin A. Experiments on a quantitative probe of the stereochemical mechanism of hemoglobin cooperativity. Biochemistry. 1979 Dec 25;18(26):5826–5833. doi: 10.1021/bi00593a012. [DOI] [PubMed] [Google Scholar]
- DeYoung A., Pennelly R. R., Tan-Wilson A. L., Noble R. W. Kinetic studies on the binding affinity of human hemoglobin for the 4th carbon monoxide molecule, L4. J Biol Chem. 1976 Nov 10;251(21):6692–6698. [PubMed] [Google Scholar]
- Edelstein S. J., Rehmar M. J., Olson J. S., Gibson Q. H. Functional aspects of the subunit association-dissociation equilibria of hemoglobin. J Biol Chem. 1970 Sep 10;245(17):4372–4381. [PubMed] [Google Scholar]
- Ferrone F. A., Hopfield J. J. Rate of quaternary structure change in hemoglobin measured by modulated excitation. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4497–4501. doi: 10.1073/pnas.73.12.4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON Q. H. The photochemical formation of a quickly reacting form of haemoglobin. Biochem J. 1959 Feb;71(2):293–303. doi: 10.1042/bj0710293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelin B. R., Karplus M. Mechanism of tertiary structural change in hemoglobin. Proc Natl Acad Sci U S A. 1977 Mar;74(3):801–805. doi: 10.1073/pnas.74.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelin B. R., Lee A. W., Karplus M. Hemoglobin tertiary structural change on ligand binding. Its role in the co-operative mechanism. J Mol Biol. 1983 Dec 25;171(4):489–559. doi: 10.1016/0022-2836(83)90042-6. [DOI] [PubMed] [Google Scholar]
- Giardina B., Ascoli F., Brunori M. Spectral changes and allosteric transition in trout haemoglobin. Nature. 1975 Aug 28;256(5520):761–762. doi: 10.1038/256761a0. [DOI] [PubMed] [Google Scholar]
- Hofrichter J., Sommer J. H., Henry E. R., Eaton W. A. Nanosecond absorption spectroscopy of hemoglobin: elementary processes in kinetic cooperativity. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2235–2239. doi: 10.1073/pnas.80.8.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfield J. J., Shulman R. G., Ogawa S. An allosteric model of hemoglobin. I. Kinetics. J Mol Biol. 1971 Oct 28;61(2):425–443. doi: 10.1016/0022-2836(71)90391-3. [DOI] [PubMed] [Google Scholar]
- Imai K., Yonetani T. PH dependence of the Adair constants of human hemoglobin. Nonuniform contribution of successive oxygen bindings to the alkaline Bohr effect. J Biol Chem. 1975 Mar 25;250(6):2227–2231. [PubMed] [Google Scholar]
- Johnson M. L., Ackers G. K. Thermodynamic analysis of human hemoglobins in terms of the Perutz mechanism: extensions of the Szabo--Karplus model to include subunit assembly. Biochemistry. 1982 Jan 19;21(2):201–211. doi: 10.1021/bi00531a001. [DOI] [PubMed] [Google Scholar]
- Johnson M. L., Turner B. W., Ackers G. K. A quantitative model for the cooperative mechanism of human hemoglobin. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1093–1097. doi: 10.1073/pnas.81.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles F. C., McDonald M. J., Gibson Q. H. The origin of the Adams-Schuster difference spectrum of oxyhemoglobin. Biochem Biophys Res Commun. 1975 Sep 16;66(2):556–563. doi: 10.1016/0006-291x(75)90546-x. [DOI] [PubMed] [Google Scholar]
- Lee A. W., Karplus M. Structure-specific model of hemoglobin cooperativity. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7055–7059. doi: 10.1073/pnas.80.23.7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson J. S. Spectral differences between the alpha and beta heme groups within human deoxyhemoglobin. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1140–1144. doi: 10.1073/pnas.73.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz M. F., Kilmartin J. V., Nagai K., Szabo A., Simon S. R. Influence of globin structures on the state of the heme. Ferrous low spin derivatives. Biochemistry. 1976 Jan 27;15(2):378–387. doi: 10.1021/bi00647a022. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Ladner J. E., Simon S. R., Ho C. Influence of globin structure on the state of the heme. I. Human deoxyhemoglobin. Biochemistry. 1974 May 7;13(10):2163–2173. doi: 10.1021/bi00707a026. [DOI] [PubMed] [Google Scholar]
- Sawicki C. A., Gibson Q. H. Quaternary conformational changes in human hemoglobin studied by laser photolysis of carboxyhemoglobin. J Biol Chem. 1976 Mar 25;251(6):1533–1542. [PubMed] [Google Scholar]
- Sawicki C. A., Gibson Q. H. Quaternary conformational changes in human oxyhemoglobin studied by laser photolysis. J Biol Chem. 1977 Aug 25;252(16):5783–5788. [PubMed] [Google Scholar]
- Sawicki C. A., Gibson Q. H. Tetramer-dimer dissociation of carboxyhemoglobin in the absence of dithionite. Biophys J. 1981 Aug;35(2):265–270. doi: 10.1016/S0006-3495(81)84788-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman R. G., Hopfield J. J., Ogawa S. Allosteric interpretation of haemoglobin properties. Q Rev Biophys. 1975 Jul;8(3):325–420. doi: 10.1017/s0033583500001840. [DOI] [PubMed] [Google Scholar]
- Sugita Y. Differences in spectra of alpha and beta chains of hemoglobin between isolated state and in tetramer. J Biol Chem. 1975 Feb 25;250(4):1251–1256. [PubMed] [Google Scholar]
- Szabo A., Karplus M. A mathematical model for structure-function relations in hemoglobin. J Mol Biol. 1972 Dec 14;72(1):163–197. doi: 10.1016/0022-2836(72)90077-0. [DOI] [PubMed] [Google Scholar]
- Tan A. L., Noble R. W. The effect of inositol hexaphosphate on the allosteric properties of carp hemoglobin. J Biol Chem. 1973 Nov 10;248(21):7412–7416. [PubMed] [Google Scholar]
- Williams R. C., Jr, Tsay K. Y. A convenient chromatographic method for the preparation of human hemoglobin. Anal Biochem. 1973 Jul;54(1):137–145. doi: 10.1016/0003-2697(73)90256-x. [DOI] [PubMed] [Google Scholar]
- Wyman J., Gill S. J., Gaud H. T., Colosimo A., Giardina B., Kupier H. A., Brunori M. Thermodynamics of ligand binding and allosteric transition in hemoglobins. Reaction of Hb trout IV with CO. J Mol Biol. 1978 Sep 5;124(1):161–175. doi: 10.1016/0022-2836(78)90154-7. [DOI] [PubMed] [Google Scholar]



