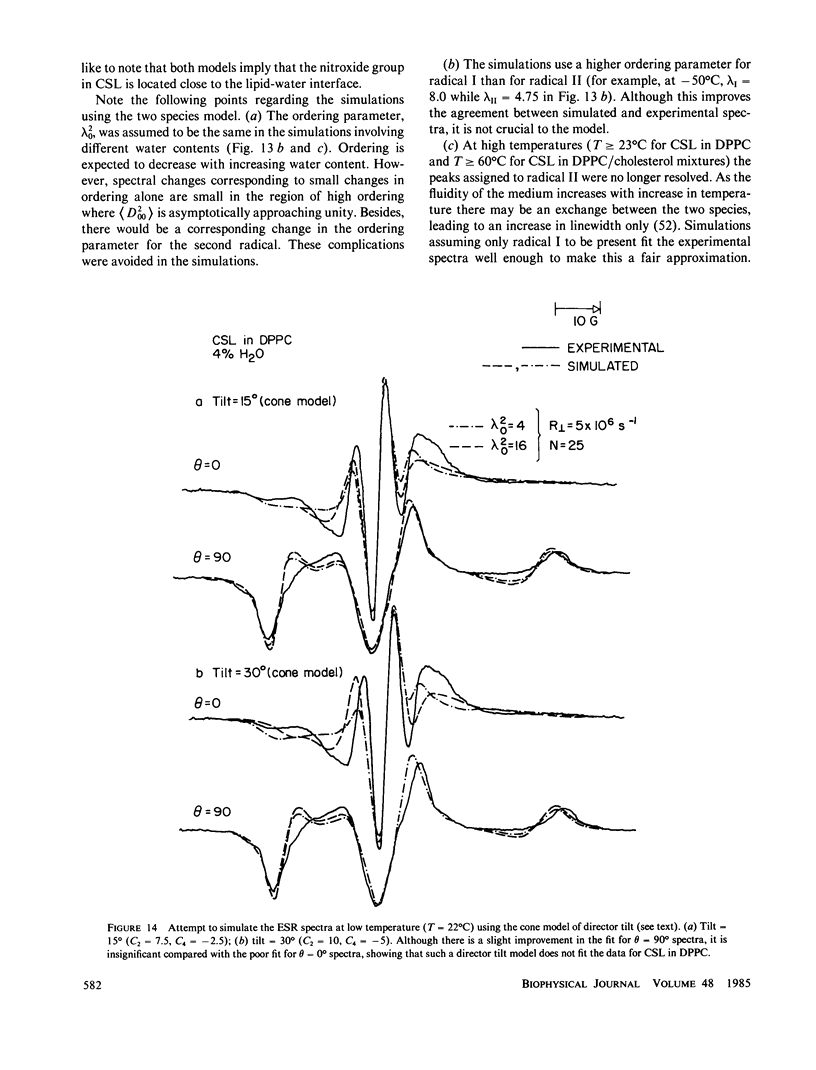
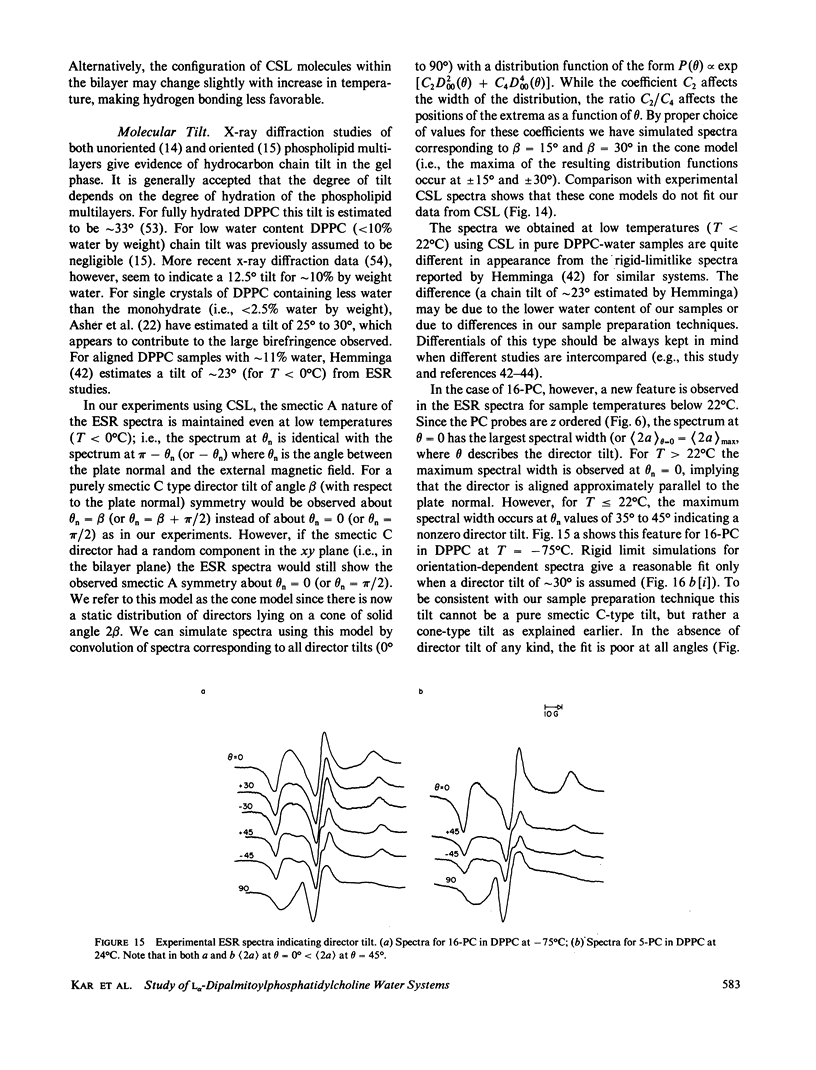
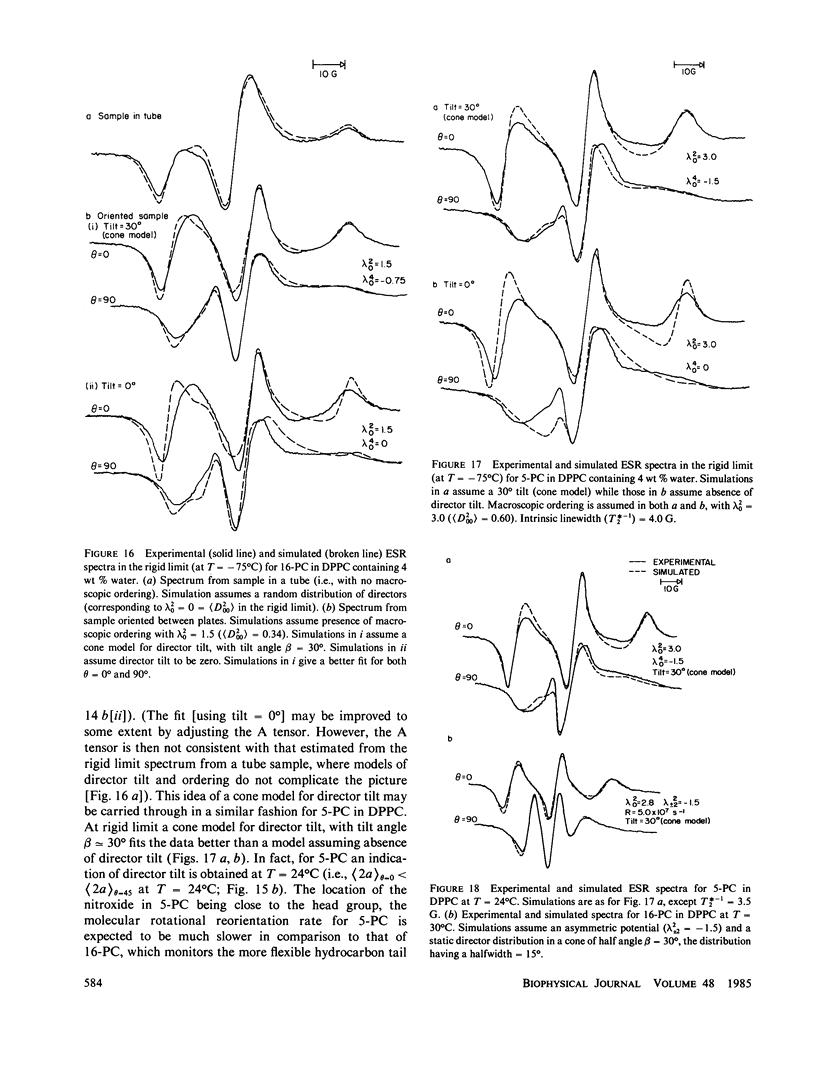
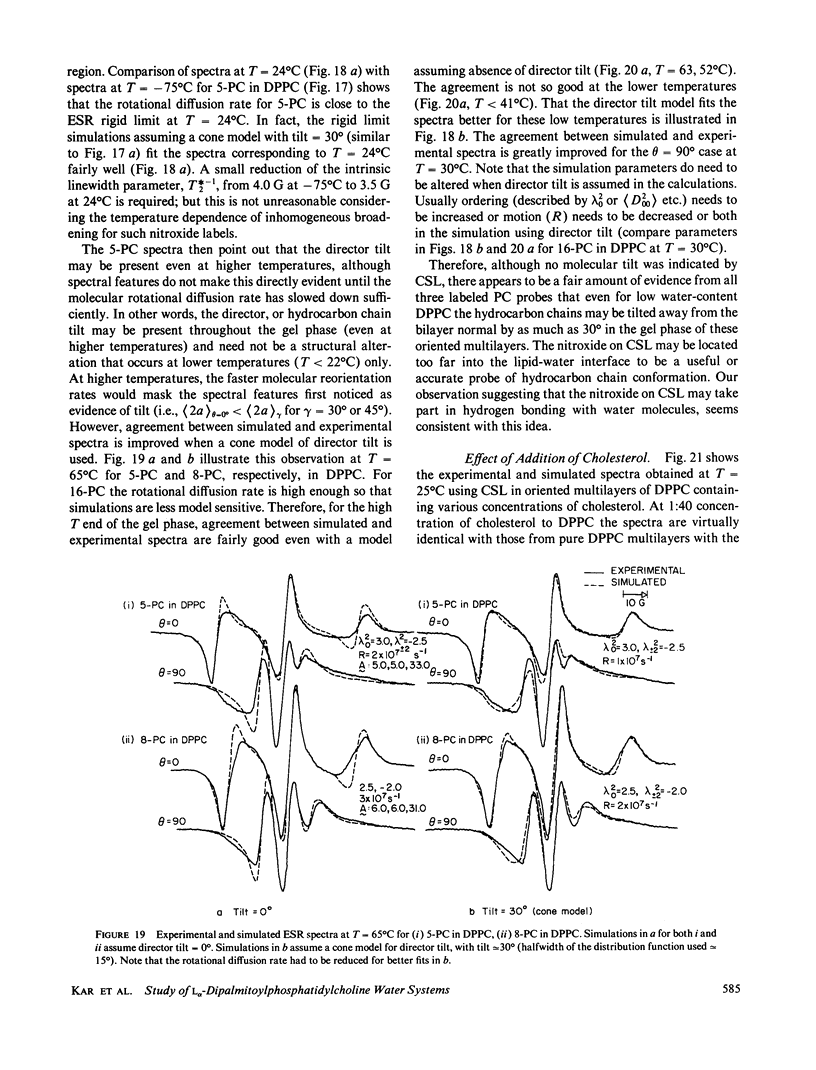
Abstract
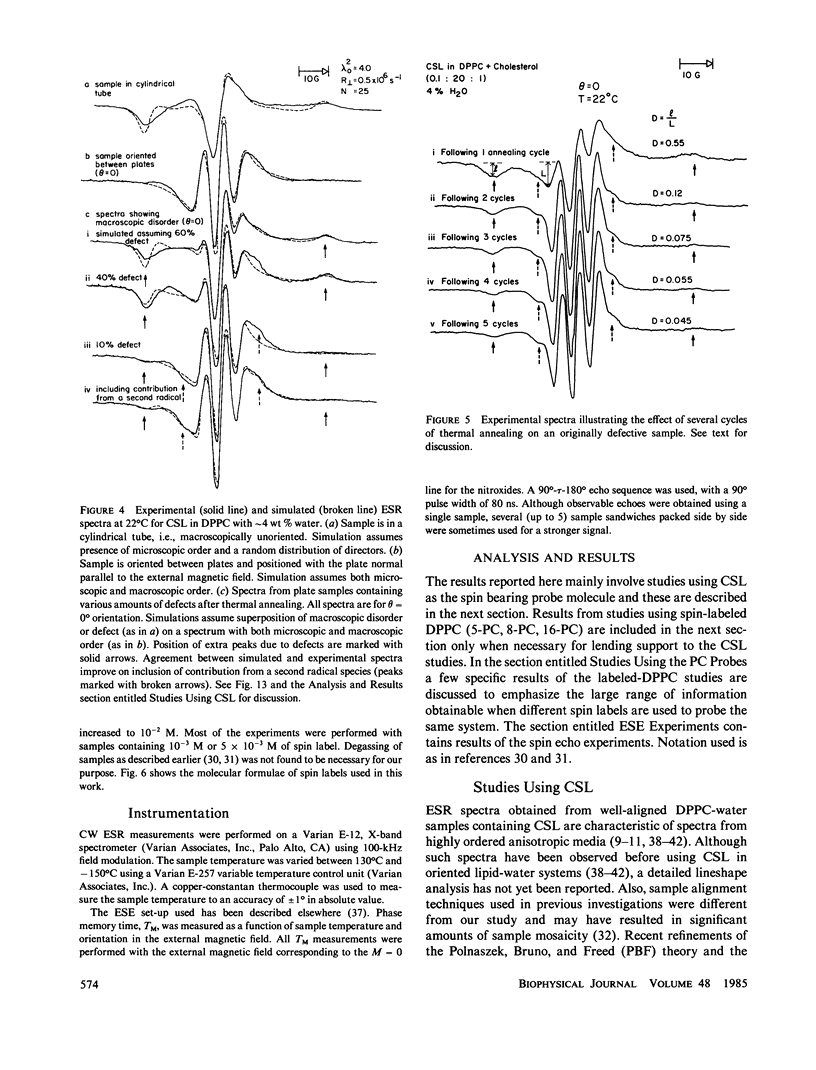
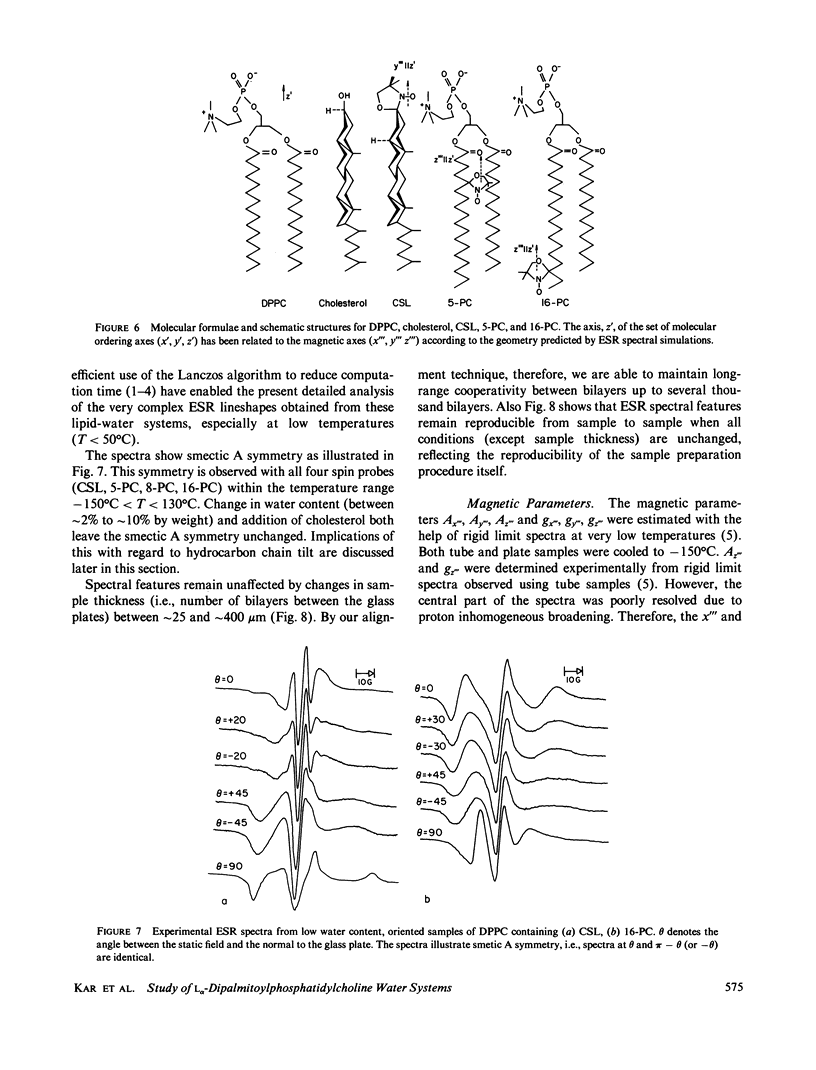
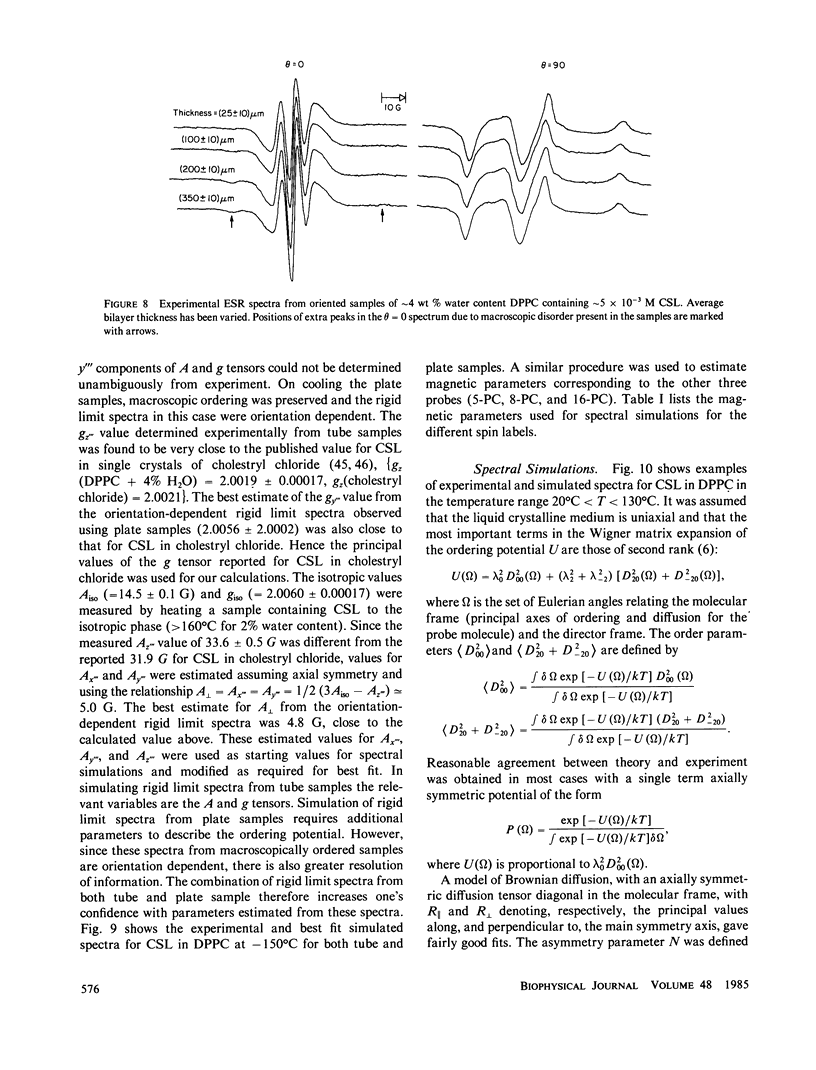
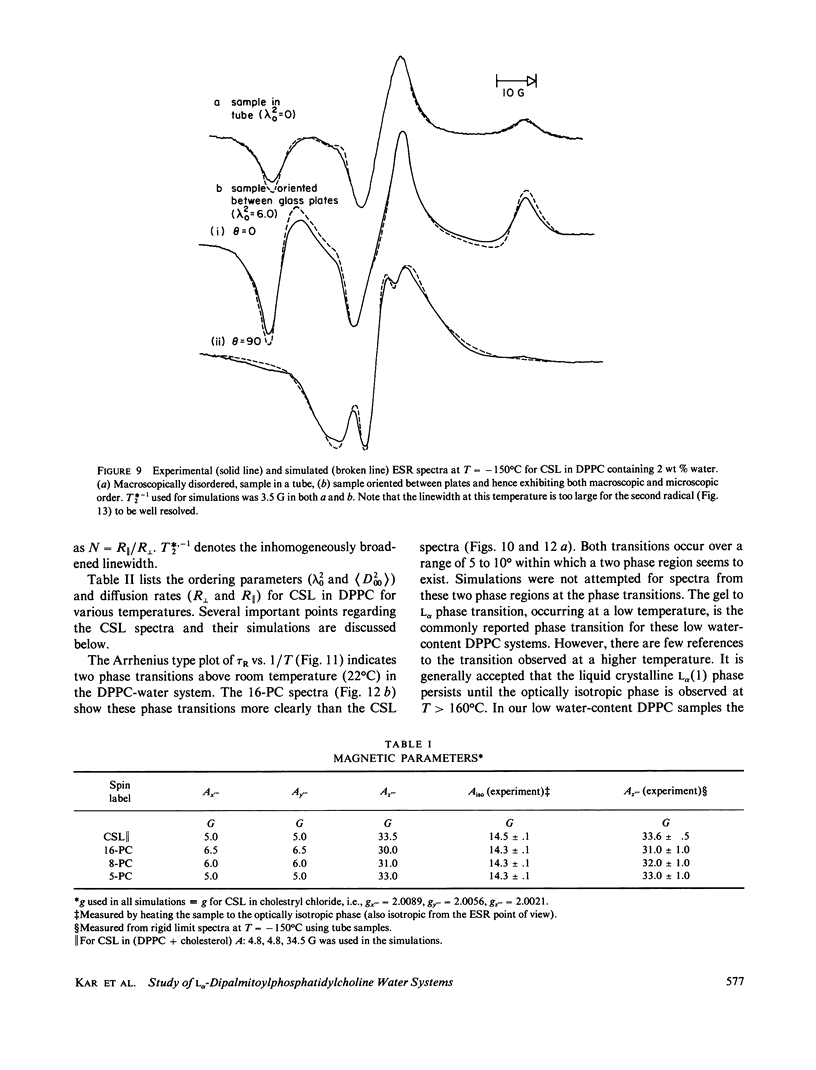
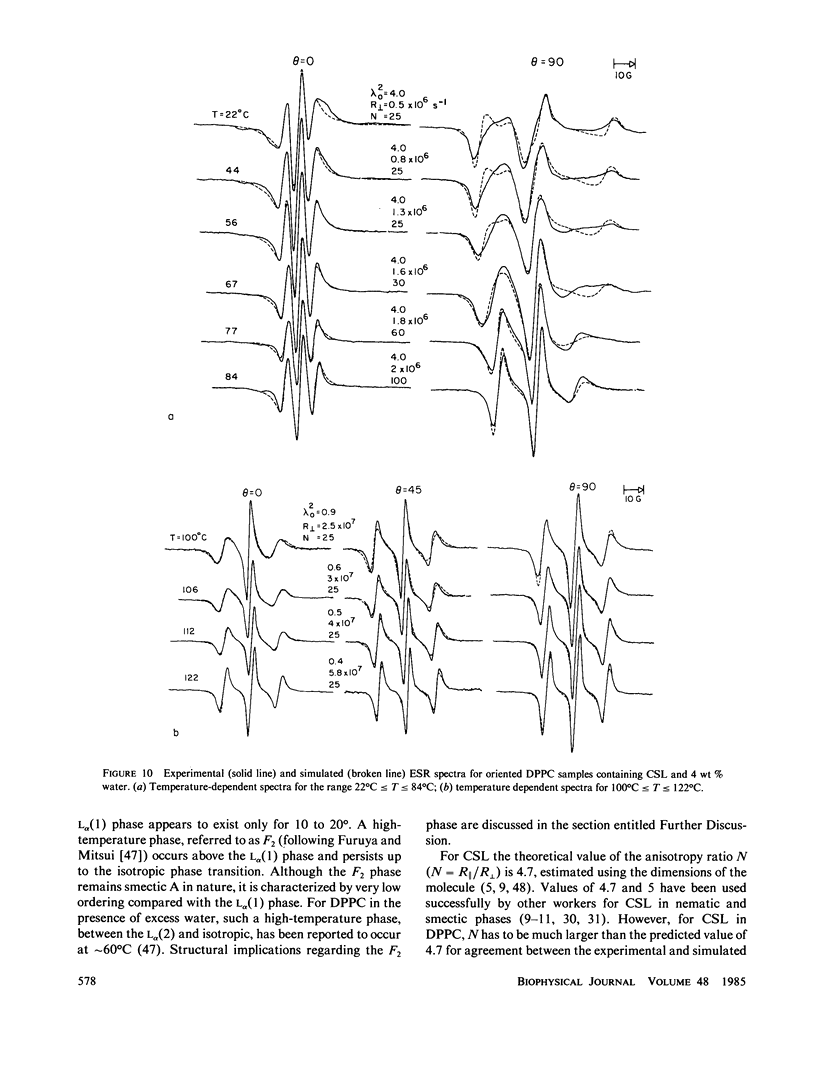
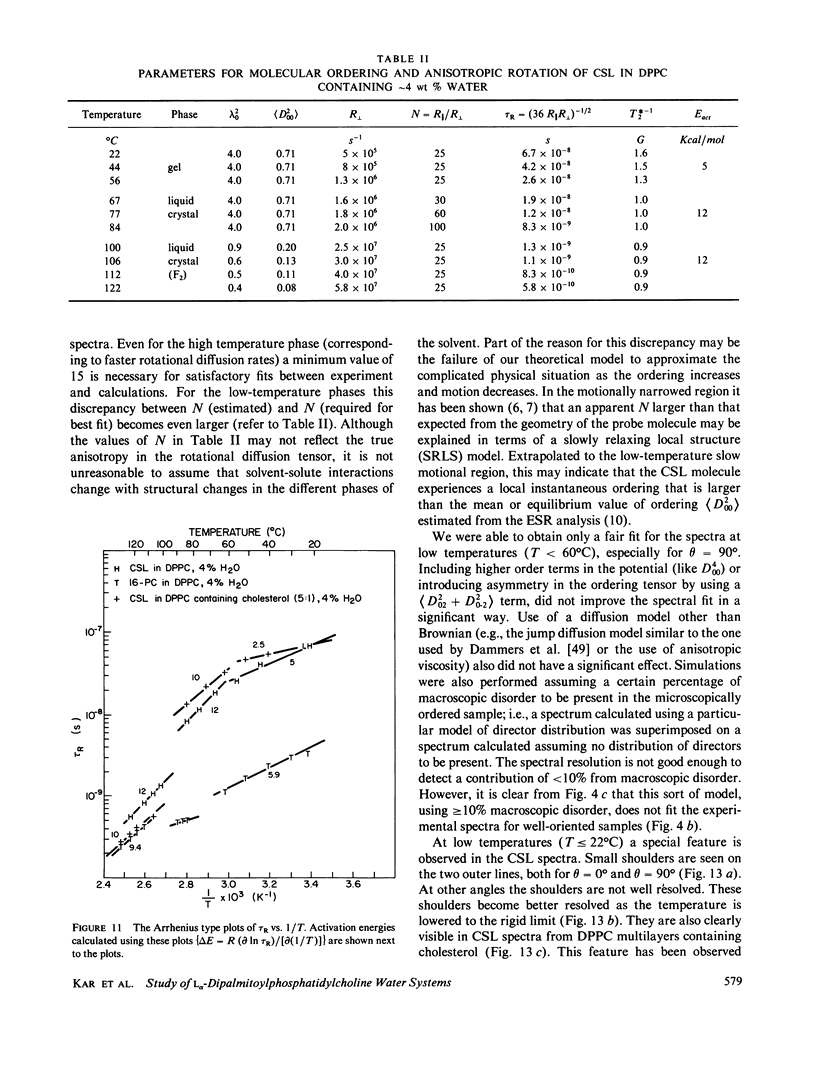
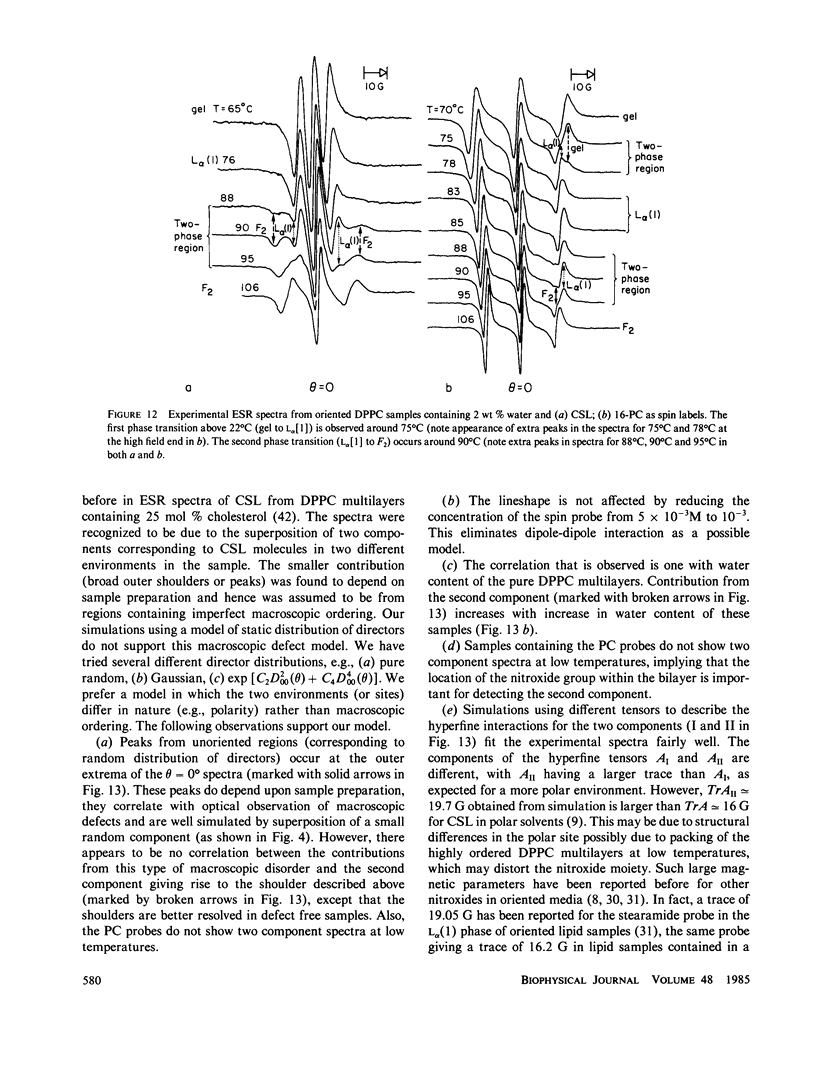
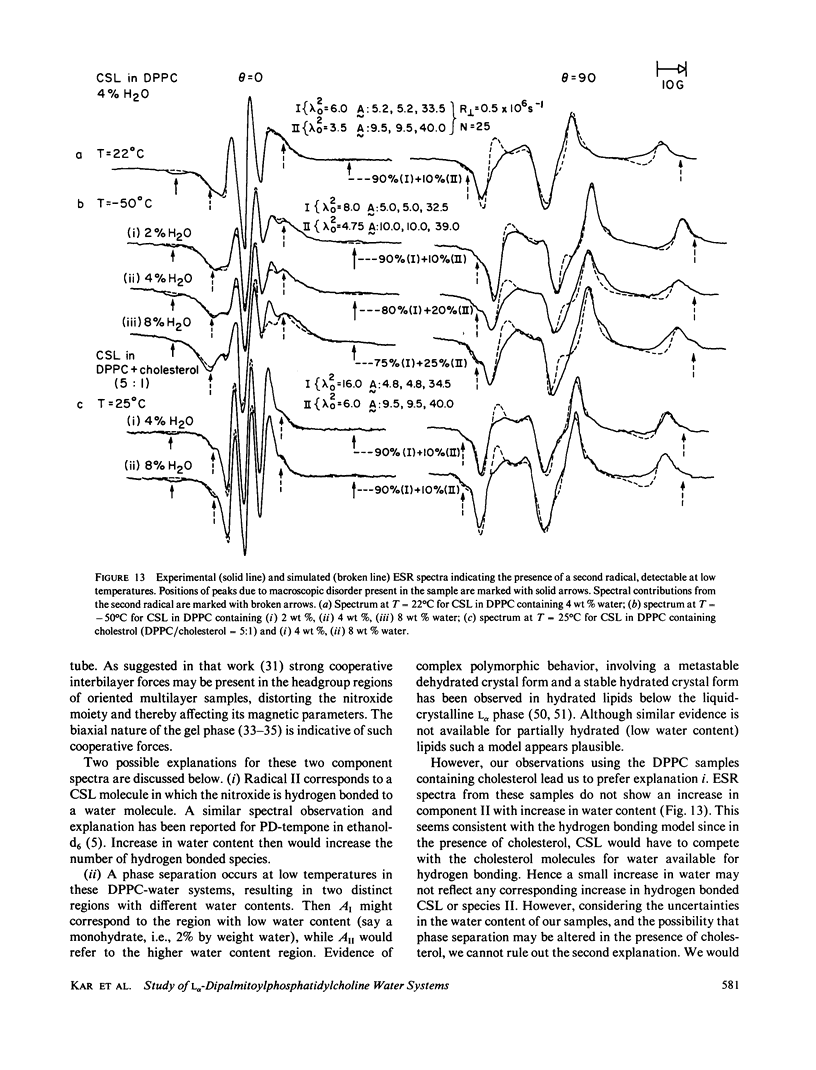
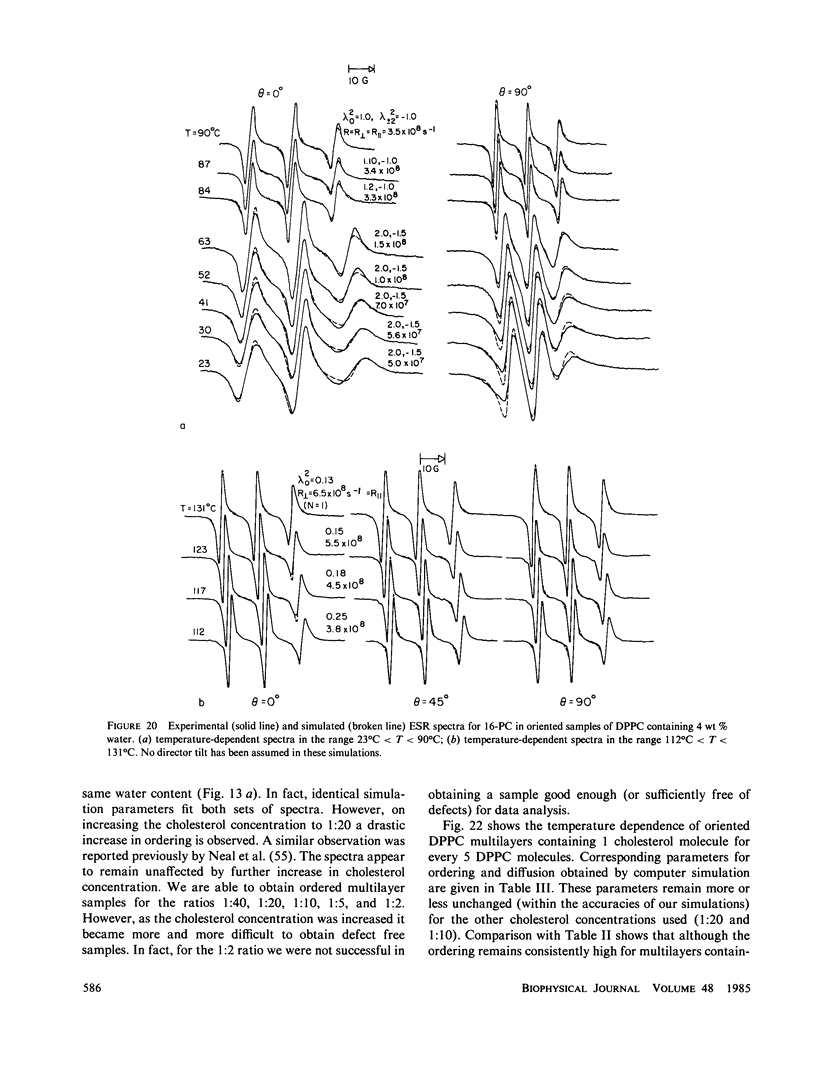
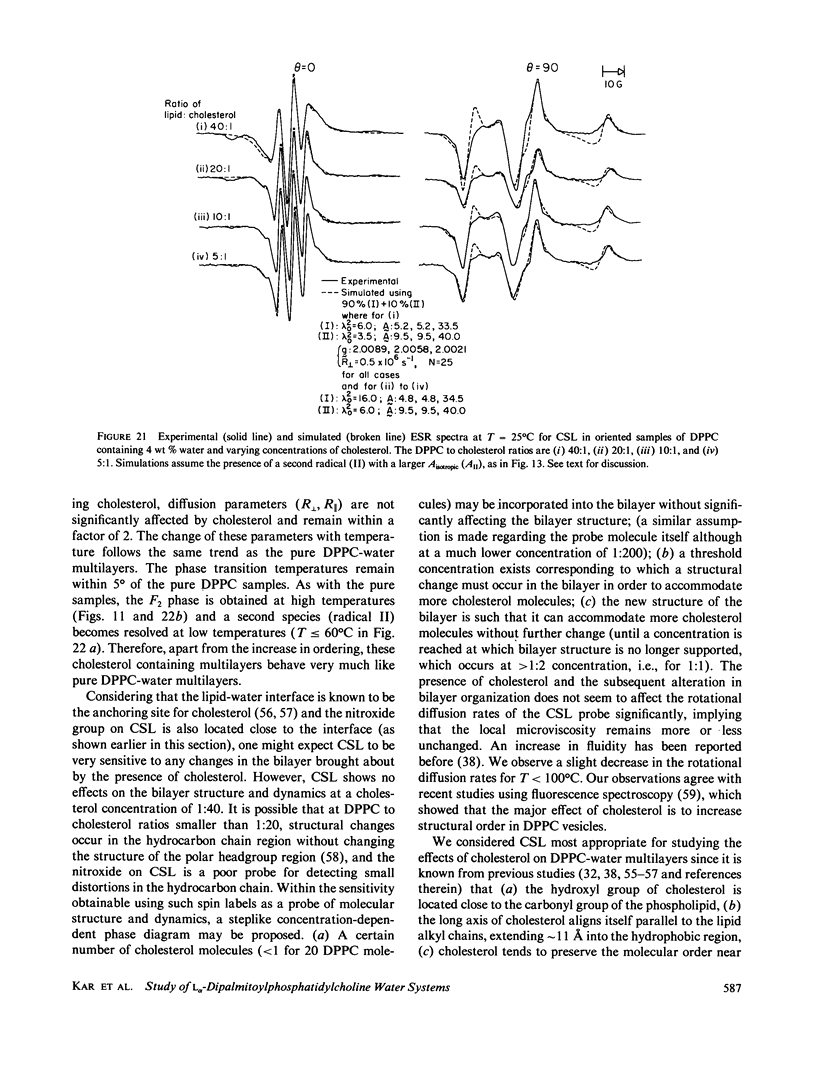
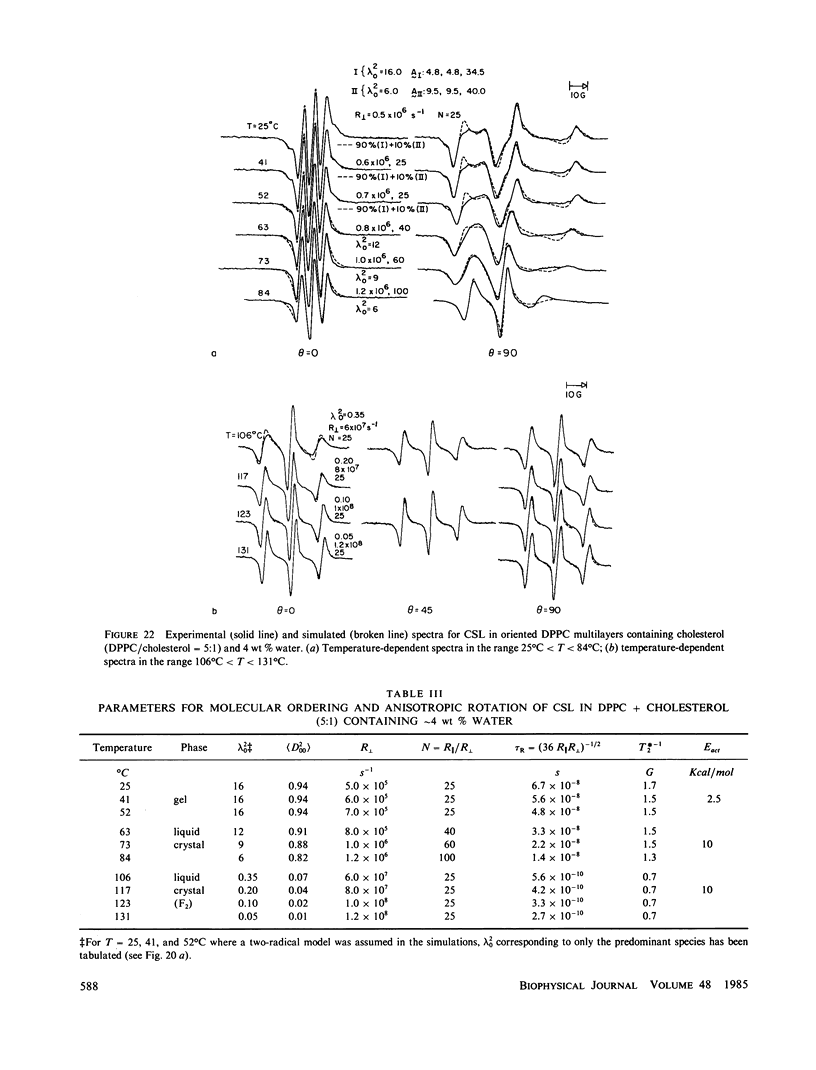
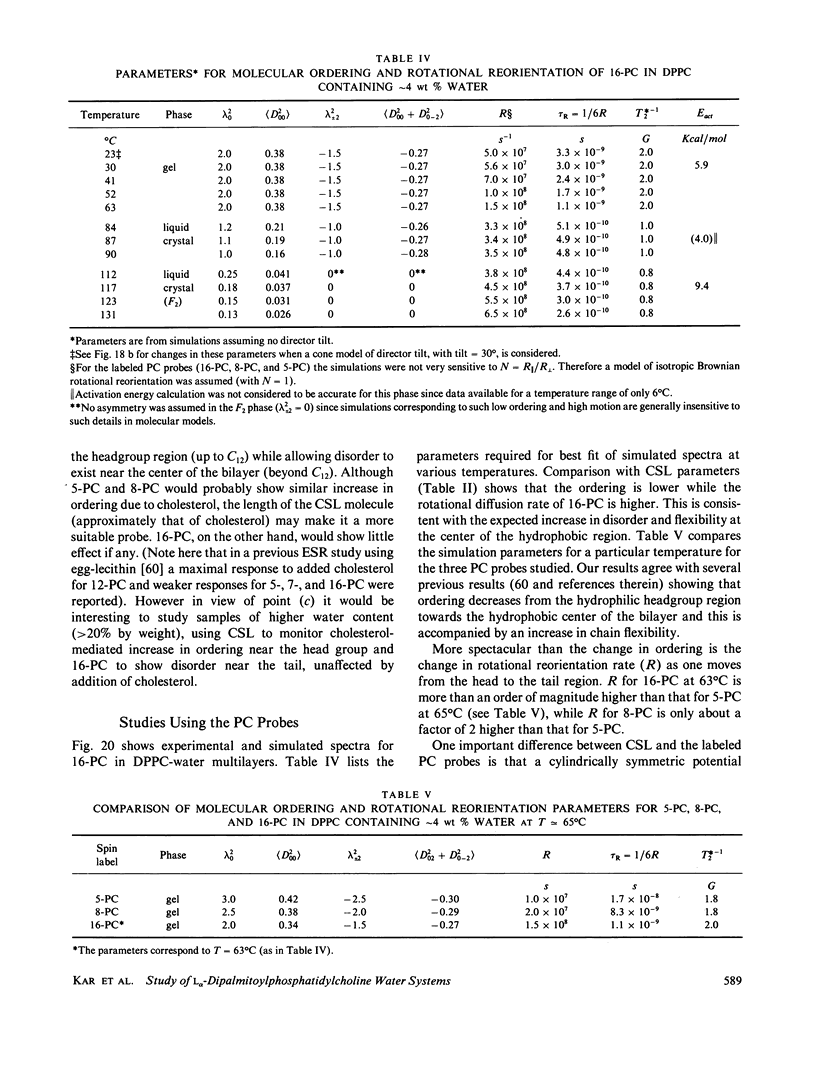
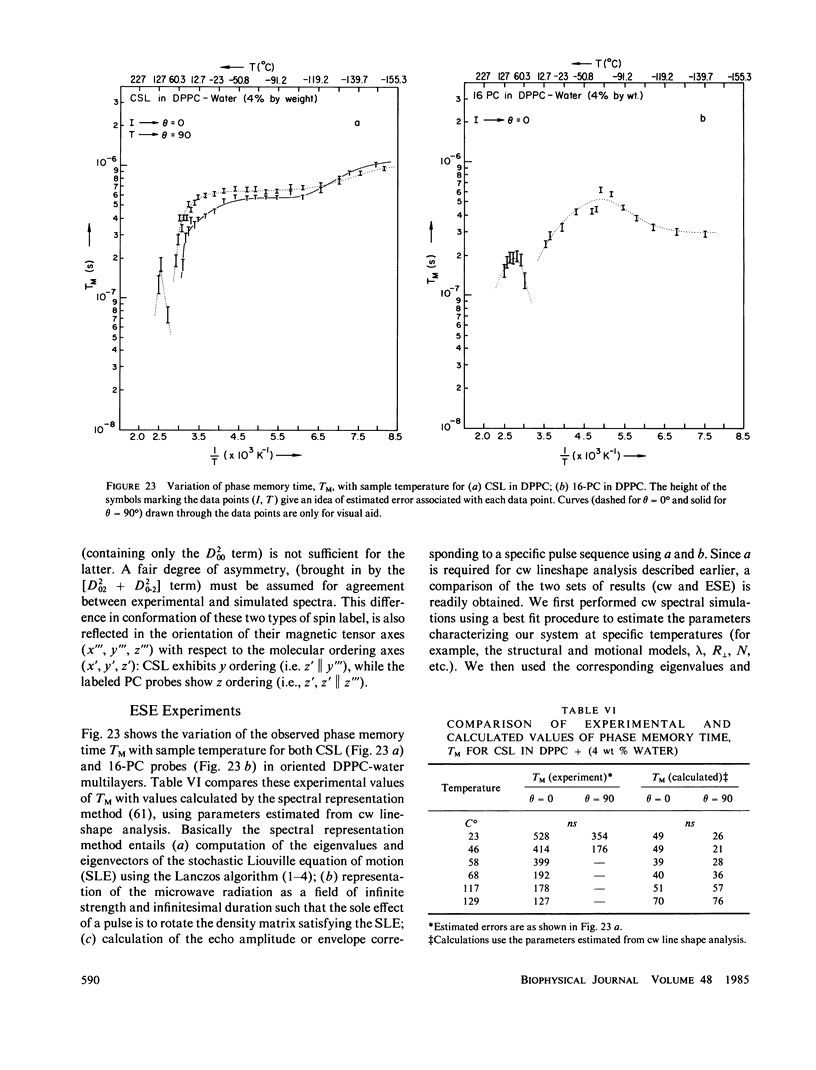
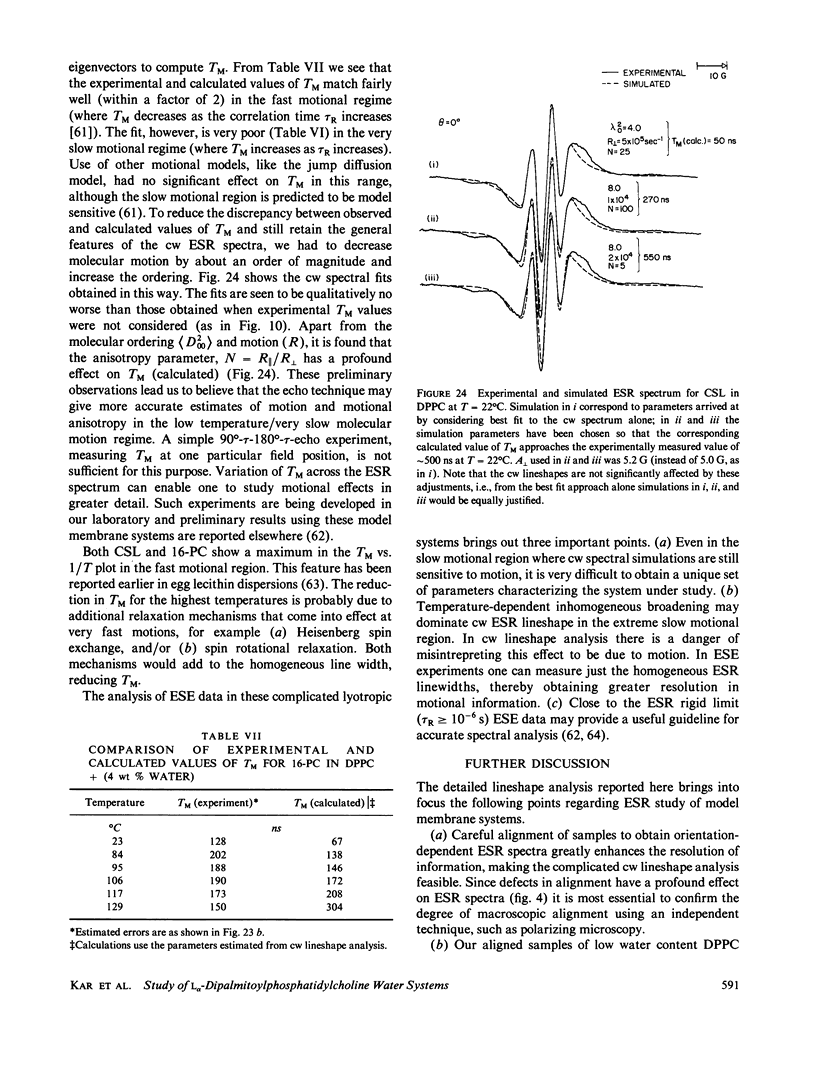
A detailed electron spin resonance (ESR) study of spin-labeled-oriented multilayers of L alpha-dipalmitoylphosphatidylcholine (DPPC) water systems for low water content (2-10% by weight) is reported with the purpose of characterizing the dynamical and structural properties of model membrane systems. Emphasis is placed on the value of combining such experiments with detailed simulations based on current slow-motional theories. Information is obtained regarding ordering and anisotropic rotational diffusion rates via ESR lineshape analysis over the entire motional range, from the fast motional region through the moderately slow and slow to the rigid limit. This includes the low-temperature gel phase, the liquid crystalline L alpha (1) phase and what appears to be a third high-temperature phase above the L alpha phase. Cholestane (CSL) and spin-labeled DPPC (5-PC, 8-PC, and 16-PC) have been used to probe different depths of the bilayer. While CSL and 5-PC both reflect the high ordering of the bilayer close to the lipid-water interface, CSL appears to be located close enough to the water for the nitroxide to be involved in hydrogen bonding with water molecules. 16-PC reflects the relatively low ordering near the tail of the hydrocarbon chain in the bilayer. Quantitative estimates of ordering and motion are obtained for these cases. The results from CSL indicate that close to the lipid-water interface the DPPC molecule is oriented approximately perpendicular to the bilayer in these low water-content systems. However, all three labeled lipid probes indicate that the hydrocarbon chain of DPPC may be bent away from the bilayer normal by as much as 30 degrees and this evidence is stronger at low temperatures. When cholesterol is added to the DPPC-water system at a concentration greater than or equal to 2.5 mol %, the ordering is greatly increased although the rotational diffusion rate remains almost unaffected in the gel phase. Electron spin echoes (ESE) are observed for the first time from oriented lipid-water multilayers. Results obtained from cw ESR lineshape analysis are correlated with data from ESE experiments, which give a more direct measurement of relaxation times. These results indicate that for detection of very slow motions (close to the rigid limit) ESE experiments are more sensitive to dynamics than continuous wave ESR for which inhomogeneous broadening becomes a major problem.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asher S. A., Pershan P. S. Alignment and defect structures in oriented phosphatidylcholine multilayers. Biophys J. 1979 Sep;27(3):393–421. doi: 10.1016/S0006-3495(79)85225-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büldt G., Gally H. U., Seelig A., Seelig J., Zaccai G. Neutron diffraction studies on selectively deuterated phospholipid bilayers. Nature. 1978 Jan 12;271(5641):182–184. doi: 10.1038/271182a0. [DOI] [PubMed] [Google Scholar]
- Chen S. C., Sturtevant J. M., Gaffney B. J. Scanning calorimetric evidence for a third phase transition in phosphatidylcholine bilayers. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5060–5063. doi: 10.1073/pnas.77.9.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demel R. A., De Kruyff B. The function of sterols in membranes. Biochim Biophys Acta. 1976 Oct 26;457(2):109–132. doi: 10.1016/0304-4157(76)90008-3. [DOI] [PubMed] [Google Scholar]
- Forslind E., Kjellander R. A structure model for the lecithin-cholesterol-water membrane. J Theor Biol. 1975 May;51(1):97–109. doi: 10.1016/0022-5193(75)90141-1. [DOI] [PubMed] [Google Scholar]
- Guyer W., Bloch K. Phosphatidylcholine and cholesterol interactions in model membranes. Chem Phys Lipids. 1983 Nov;33(4):313–322. doi: 10.1016/0009-3084(83)90025-7. [DOI] [PubMed] [Google Scholar]
- Hubbell W. L., McConnell H. M. Molecular motion in spin-labeled phospholipids and membranes. J Am Chem Soc. 1971 Jan 27;93(2):314–326. doi: 10.1021/ja00731a005. [DOI] [PubMed] [Google Scholar]
- Janiak M. J., Small D. M., Shipley G. G. Nature of the Thermal pretransition of synthetic phospholipids: dimyristolyl- and dipalmitoyllecithin. Biochemistry. 1976 Oct 19;15(21):4575–4580. doi: 10.1021/bi00666a005. [DOI] [PubMed] [Google Scholar]
- Johnson M. E. Apparent hydrogen bonding by strongly immobilized spin-labels. Biochemistry. 1981 Jun 9;20(12):3319–3328. doi: 10.1021/bi00515a001. [DOI] [PubMed] [Google Scholar]
- Jost P. C., Griffith O. H. The molecular reorganization of lipid bilayers by osmium tetroxide. A spin-label study of orientation and restricted y-axis anisotropic motion in model membrane systems. Arch Biochem Biophys. 1973 Nov;159(1):70–81. doi: 10.1016/0003-9861(73)90430-x. [DOI] [PubMed] [Google Scholar]
- Levine Y. K. Physical studies of membrane structure. Prog Biophys Mol Biol. 1972;24:1–74. doi: 10.1016/0079-6107(72)90003-x. [DOI] [PubMed] [Google Scholar]
- Luna E. J., McConnell H. M. The intermediate monoclinic phase of phosphatidylcholines. Biochim Biophys Acta. 1977 May 2;466(3):381–392. doi: 10.1016/0005-2736(77)90331-5. [DOI] [PubMed] [Google Scholar]
- Marriott T. B., Birrell G. B., Griffith O. H. Assignment of the configuration of the steroid spin label, 3-doxyl-5alpha-cholestane. J Am Chem Soc. 1975 Feb 5;97(3):627–630. doi: 10.1021/ja00836a026. [DOI] [PubMed] [Google Scholar]
- Marsh D. Electron spin resonance: spin labels. Mol Biol Biochem Biophys. 1981;31:51–142. doi: 10.1007/978-3-642-81537-9_2. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J. The effect of cholesterol on the structure of phosphatidylcholine bilayers. Biochim Biophys Acta. 1978 Oct 19;513(1):43–58. doi: 10.1016/0005-2736(78)90110-4. [DOI] [PubMed] [Google Scholar]
- Neal M. J., Butler K. W., Polnaszek C. F., Smith I. C. The influence of anesthetics and cholesterol on the degree of molecular organization and mobility of ox brain white matter. Lipids in multibilayer membranes: a spin probe study using spectral simulation by the stochastic method. Mol Pharmacol. 1976 Jan;12(1):144–155. [PubMed] [Google Scholar]
- Powers L., Clark N. A. Preparation of large monodomain phospholipid bilayer smectic liquid crystals. Proc Natl Acad Sci U S A. 1975 Mar;72(3):840–843. doi: 10.1073/pnas.72.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruocco M. J., Atkinson D., Small D. M., Skarjune R. P., Oldfield E., Shipley G. G. X-ray diffraction and calorimetric study of anhydrous and hydrated N-palmitoylgalactosylsphingosine (cerebroside). Biochemistry. 1981 Oct 13;20(21):5957–5966. doi: 10.1021/bi00524a006. [DOI] [PubMed] [Google Scholar]
- Schreier-Muccillo S., Marsh D., Dugas H., Schneider H., Smith C. P. A spin probe study of the influence of cholesterol on motion and orientation of phospholipids in oriented multibilayers and vesicles. Chem Phys Lipids. 1973 Jan;10(1):11–27. doi: 10.1016/0009-3084(73)90037-6. [DOI] [PubMed] [Google Scholar]
- Schreier S., Polnaszek C. F., Smith I. C. Spin labels in membranes. Problems in practice. Biochim Biophys Acta. 1978 Dec 15;515(4):395–436. doi: 10.1016/0304-4157(78)90011-4. [DOI] [PubMed] [Google Scholar]
- Shimoyama Y., Eriksson L. E., Ehrenberg A. Molecular motion and order in oriented lipid multibilayer membranes evaluated by simulations of spin label ESR spectra. Effects of temperature, cholesterol and magnetic field. Biochim Biophys Acta. 1978 Apr 4;508(2):213–235. doi: 10.1016/0005-2736(78)90326-7. [DOI] [PubMed] [Google Scholar]
- Stamatoff J. B., Graddick W. F., Powers L., Moncton D. E. Direct observation of the hydrocarbon chain tilt angle in phospholipid bilayers. Biophys J. 1979 Feb;25(2 Pt 1):253–261. doi: 10.1016/s0006-3495(79)85289-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieu A., Luzzati V., Reman F. C. Structure and polymorphism of the hydrocarbon chains of lipids: a study of lecithin-water phases. J Mol Biol. 1973 Apr 25;75(4):711–733. doi: 10.1016/0022-2836(73)90303-3. [DOI] [PubMed] [Google Scholar]
- Taylor M. G., Smith I. C. The fidelity of response by nitroxide spin probes to changes in membrane organization: the condensing effect of cholesterol. Biochim Biophys Acta. 1980 Jun 20;599(1):140–149. doi: 10.1016/0005-2736(80)90063-2. [DOI] [PubMed] [Google Scholar]
- Trahms L., Klabe W. D., Boroske E. 1H-NMR study of the three low temperature phases of DPPC-water systems. Biophys J. 1983 Jun;42(3):285–293. doi: 10.1016/S0006-3495(83)84396-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worcester D. L., Franks N. P. Structural analysis of hydrated egg lecithin and cholesterol bilayers. II. Neutrol diffraction. J Mol Biol. 1976 Jan 25;100(3):359–378. doi: 10.1016/s0022-2836(76)80068-x. [DOI] [PubMed] [Google Scholar]
- Yellin N., Levin I. W. Hydrocarbon chain disorder in lipid bilayers. Temperature dependent Raman spectra of 1,2-diacyl phosphatidylcholine-water gels. Biochim Biophys Acta. 1977 Nov 24;489(2):177–190. doi: 10.1016/0005-2760(77)90137-0. [DOI] [PubMed] [Google Scholar]