Abstract
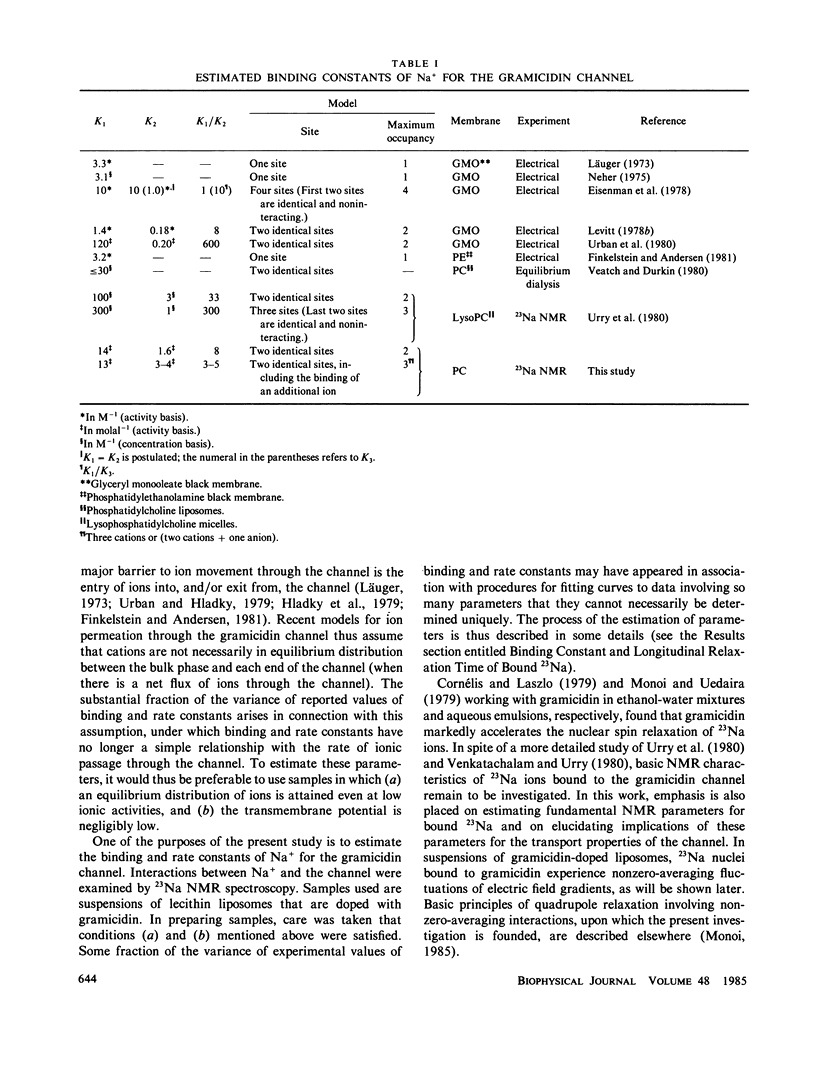
Basic nuclear magnetic resonance (NMR) features of 23Na ions bound to the gramicidin channel (packaged into lecithin liposomes) were studied. The first binding constant K1 of Na+ was not significantly dependent on channel models employed. With the two-identical-site model (Model I), K1 was 13.7 (+/- 1.4) molal-1 (in the activity basis) at 25 degrees C; when the binding of a third ion was included (Model II), it was 13.0 (+/- 2.0) molal-1. The second binding constant K2 was model dependent; it was 1.6 (+/- 0.2) and 3-4 molal-1 for Models I and II, respectively. The rate constants, k-1 and k-2, of Na+ for exit from singly and doubly loaded channels, respectively, were 8 X 10(5) s-1 less than or equal to k-1 less than or equal to 3 X 10(6) s-1 and 8 X 10(5) s-1 less than or equal to k-2 less than or equal to 1.0 X 10(7) s-1 at 25 degrees C; the lower bound represents a rough approximation of k-1. The ratio k-2/k-1 was greater than one and did not greatly exceed 20. From the competition experiment, K1 of T1+ was 5.7 (+/- 0.6) X 10(2) molal-1. The longitudinal relaxation time T1 of bound 23Na in the state of single occupancy (T 1B sing) was virtually independent of models, 0.56 (+/- 0.03) and 0.55 (+/- 0.04) ms at 25 degrees C for Models I and II, respectively. For the state of double occupancy, T1 of bound 23Na (T 1B doub) was model dependent: 0.27 (+/- 0.01) and 0.4-0.6 ms for Models I and II. The correlation time tau c of bound 23Na was 2.2 (+/- 0.2) ns at 25 degrees C for single occupancy; tau c for double occupancy was not significantly different from this value. The estimated tau c was found to involve no appreciable contribution of the exchange of 23Na between the channel and the bulk solution. Thé quadrupole coupling constant chi was 1.0 (+/- 0.1) MHz for 23Na in single occupancy; chi for double occupancy was 0.9-1.4 MHz, depending on models. A lower bound of the average quadrupole coupling constant chi alpha was 0.13-0.26 MHz at 25 degrees C for 23Na in single occupancy; this value represents a rough approximation of chi alpha at this temperature. An argument based on the estimated chi alpha and the known conformation of the gramicidin channel suggests that the binding site is a small domain near the channel end. Within the framework of Model I, Tb was faster than Tljn; this inequality was attributed to an increased chi in the presence ofa second cation, which was not explained in terms of electrostatic interactions between bound cations, implying a conformation change upon binding of cations.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen O. S. Ion movement through gramicidin A channels. Interfacial polarization effects on single-channel current measurements. Biophys J. 1983 Feb;41(2):135–146. doi: 10.1016/S0006-3495(83)84415-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen O. S., Procopio J. Ion movement through gramicidin A channels. On the importance of the aqueous diffusion resistance and ion-water interactions. Acta Physiol Scand Suppl. 1980;481:27–35. [PubMed] [Google Scholar]
- Bamberg E., Apell H. J., Alpes H. Structure of the gramicidin A channel: discrimination between the piL,D and the beta helix by electrical measurements with lipid bilayer membranes. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2402–2406. doi: 10.1073/pnas.74.6.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberg E., Läuger P. Temperature-dependent properties of gramicidin A channels. Biochim Biophys Acta. 1974 Oct 29;367(2):127–133. doi: 10.1016/0005-2736(74)90037-6. [DOI] [PubMed] [Google Scholar]
- Bamberg E., Noda K., Gross E., Läuger P. Single-channel parameters of gramicidin A,B, and C. Biochim Biophys Acta. 1976 Jan 21;419(2):223–228. doi: 10.1016/0005-2736(76)90348-5. [DOI] [PubMed] [Google Scholar]
- Berendsen H. J., Edzes H. T. The observation and general interpretation of sodium magnetic resonance in biological material. Ann N Y Acad Sci. 1973 Mar 30;204:459–485. doi: 10.1111/j.1749-6632.1973.tb30799.x. [DOI] [PubMed] [Google Scholar]
- Cornélis A., Laszlo P. Sodium binding sites of gramicidin A: sodium-23 nuclear magnetic resonance study. Biochemistry. 1979 May 15;18(10):2004–2007. doi: 10.1021/bi00577a025. [DOI] [PubMed] [Google Scholar]
- Dani J. A., Levitt D. G. Binding constants of Li+, K+, and Tl+ in the gramicidin channel determined from water permeability measurements. Biophys J. 1981 Aug;35(2):485–499. doi: 10.1016/S0006-3495(81)84804-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubos R. J. STUDIES ON A BACTERICIDAL AGENT EXTRACTED FROM A SOIL BACILLUS : I. PREPARATION OF THE AGENT. ITS ACTIVITY IN VITRO. J Exp Med. 1939 Jun 30;70(1):1–10. doi: 10.1084/jem.70.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubos R. J. STUDIES ON A BACTERICIDAL AGENT EXTRACTED FROM A SOIL BACILLUS : II. PROTECTIVE EFFECT OF THE BACTERICIDAL AGENT AGAINST EXPERIMENTAL PNEUMOCOCCUS INFECTIONS IN MICE. J Exp Med. 1939 Jun 30;70(1):11–17. doi: 10.1084/jem.70.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman G., Hägglund J., Sandblom J., Enos B. The current-voltage behavior of ion channels: important features of the energy profile of the gramicidin channel deduced from the conductance-voltage characteristic in the limit of low ion concentration. Ups J Med Sci. 1980;85(3):247–257. doi: 10.3109/03009738009179195. [DOI] [PubMed] [Google Scholar]
- Eisenman G., Sandblom J., Neher E. Interactions in cation permeation through the gramicidin channel. Cs, Rb, K, Na, Li, Tl, H, and effects of anion binding. Biophys J. 1978 May;22(2):307–340. doi: 10.1016/S0006-3495(78)85491-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein A., Andersen O. S. The gramicidin A channel: a review of its permeability characteristics with special reference to the single-file aspect of transport. J Membr Biol. 1981 Apr 30;59(3):155–171. doi: 10.1007/BF01875422. [DOI] [PubMed] [Google Scholar]
- Haydon D. A. Functions of the lipid in bilayer ion permeability. Ann N Y Acad Sci. 1975 Dec 30;264:2–16. doi: 10.1111/j.1749-6632.1975.tb31472.x. [DOI] [PubMed] [Google Scholar]
- Hinton J. F., Young G., Millett F. S. Thallous ion interaction with gramicidin incorporated in micelles studied by thallium-205 nuclear magnetic resonance. Biochemistry. 1982 Feb 16;21(4):651–654. doi: 10.1021/bi00533a009. [DOI] [PubMed] [Google Scholar]
- Hladky S. B., Haydon D. A. Discreteness of conductance change in bimolecular lipid membranes in the presence of certain antibiotics. Nature. 1970 Jan 31;225(5231):451–453. doi: 10.1038/225451a0. [DOI] [PubMed] [Google Scholar]
- Hladky S. B., Haydon D. A. Ion transfer across lipid membranes in the presence of gramicidin A. I. Studies of the unit conductance channel. Biochim Biophys Acta. 1972 Aug 9;274(2):294–312. doi: 10.1016/0005-2736(72)90178-2. [DOI] [PubMed] [Google Scholar]
- Hägglund J., Enos B., Eisenman G. Multi-site, multi-barrier, multi-occupancy models for the electrical behavior of single filing channels like those of gramicidin. Brain Res Bull. 1979 Jan-Feb;4(1):154–158. doi: 10.1016/0361-9230(79)90077-7. [DOI] [PubMed] [Google Scholar]
- Jordan P. C. Electrostatic modeling of ion pores. II. Effects attributable to the membrane dipole potential. Biophys J. 1983 Feb;41(2):189–195. doi: 10.1016/S0006-3495(83)84419-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeppe R. E., 2nd, Berg J. M., Hodgson K. O., Stryer L. Gramicidin A crystals contain two cation binding sites per channel. Nature. 1979 Jun 21;279(5715):723–725. doi: 10.1038/279723a0. [DOI] [PubMed] [Google Scholar]
- Kolb H. A., Bamberg E. Influence of membrane thickness and ion concentration on the properties of the gramicidin a channel. Autocorrelation, spectral power density, relaxation and single-channel studies. Biochim Biophys Acta. 1977 Jan 4;464(1):127–141. doi: 10.1016/0005-2736(77)90376-5. [DOI] [PubMed] [Google Scholar]
- Krasne S., Eisenman G., Szabo G. Freezing and melting of lipid bilayers and the mode of action of nonactin, valinomycin, and gramicidin. Science. 1971 Oct 22;174(4007):412–415. doi: 10.1126/science.174.4007.412. [DOI] [PubMed] [Google Scholar]
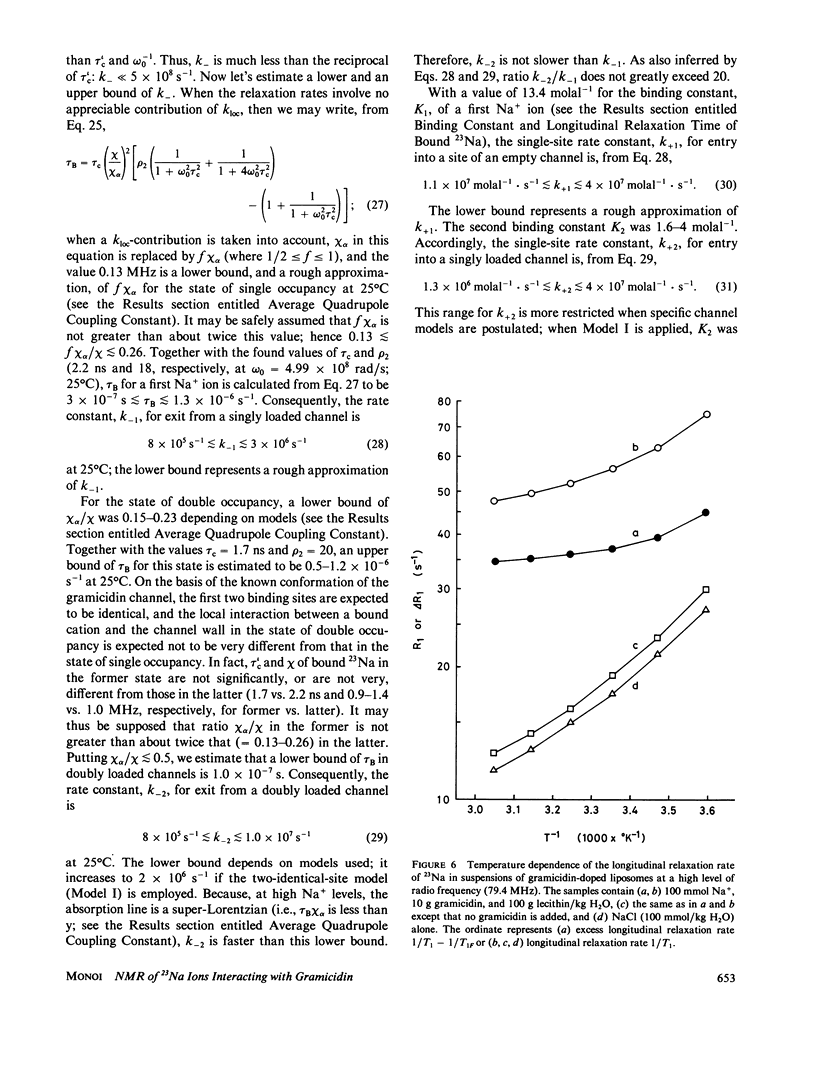
- Levitt D. G. Electrostatic calculations for an ion channel. I. Energy and potential profiles and interactions between ions. Biophys J. 1978 May;22(2):209–219. doi: 10.1016/S0006-3495(78)85485-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt D. G. Electrostatic calculations for an ion channel. II. Kinetic behavior of the gramicidin A channel. Biophys J. 1978 May;22(2):221–248. doi: 10.1016/S0006-3495(78)85486-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt D. G., Elias S. R., Hautman J. M. Number of water molecules coupled to the transport of sodium, potassium and hydrogen ions via gramicidin, nonactin or valinomycin. Biochim Biophys Acta. 1978 Sep 22;512(2):436–451. doi: 10.1016/0005-2736(78)90266-3. [DOI] [PubMed] [Google Scholar]
- Läuger P. Ion transport through pores: a rate-theory analysis. Biochim Biophys Acta. 1973 Jul 6;311(3):423–441. doi: 10.1016/0005-2736(73)90323-4. [DOI] [PubMed] [Google Scholar]
- Läuger P. Kinetic properties of ion carriers and channels. J Membr Biol. 1980 Dec 30;57(3):163–78(-RETURN-). doi: 10.1007/BF01869585. [DOI] [PubMed] [Google Scholar]
- Masotti L., Spisni A., Urry D. W. Conformational studies on the gramicidin A transmembrane channel in lipid micelles and liposomes. Cell Biophys. 1980 Sep;2(3):241–251. doi: 10.1007/BF02790452. [DOI] [PubMed] [Google Scholar]
- Monoi H. Ionic interactions and anion binding in the gramicidin channel. An electrostatic calculation. J Theor Biol. 1983 May 7;102(1):69–99. doi: 10.1016/0022-5193(83)90263-1. [DOI] [PubMed] [Google Scholar]
- Monoi H. Possible existence of ion pairs at the mouths of ion channels. Biochim Biophys Acta. 1982 Dec 8;693(1):159–164. doi: 10.1016/0005-2736(82)90482-5. [DOI] [PubMed] [Google Scholar]
- Monoi H., Uedaira H. Na+ interacting with gramicidin D. A nuclear magnetic resonance study. Biophys J. 1979 Mar;25(3):535–540. doi: 10.1016/S0006-3495(79)85321-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers V. B., Haydon D. A. Ion transfer across lipid membranes in the presence of gramicidin A. II. The ion selectivity. Biochim Biophys Acta. 1972 Aug 9;274(2):313–322. doi: 10.1016/0005-2736(72)90179-4. [DOI] [PubMed] [Google Scholar]
- Neher E., Sandblom J., Eisenman G. Ionic selectivity, saturation, and block in gramicidin A channels. II. Saturation behavior of single channel conductances and evidence for the existence of multiple binding sites in the channel. J Membr Biol. 1978 Apr 26;40(2):97–116. doi: 10.1007/BF01871143. [DOI] [PubMed] [Google Scholar]
- Rosenberg P. A., Finkelstein A. Interaction of ions and water in gramicidin A channels: streaming potentials across lipid bilayer membranes. J Gen Physiol. 1978 Sep;72(3):327–340. doi: 10.1085/jgp.72.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg P. A., Finkelstein A. Water permeability of gramicidin A-treated lipid bilayer membranes. J Gen Physiol. 1978 Sep;72(3):341–350. doi: 10.1085/jgp.72.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnev V. S., Ermishkin L. N., Fonina L. A., Rovin YuG The dependence of the conductance and lifetime of gramicidin channels on the thickness and tension of lipid bilayers. Biochim Biophys Acta. 1981 Mar 20;642(1):196–202. doi: 10.1016/0005-2736(81)90149-8. [DOI] [PubMed] [Google Scholar]
- Sandblom J., Eisenman G., Neher E. Ionic selectivity, saturation and block in gramicidin A channels: I. Theory for the electrical properties of ion selective channels having two pairs of binding sites and multiple conductance states. J Membr Biol. 1977 Mar 23;31(4):383–347. doi: 10.1007/BF01869414. [DOI] [PubMed] [Google Scholar]
- Schagina L. V., Grinfeldt A. E., Lev A. A. Interaction of cation fluxes in gramicidin A channels in lipid bilayer membranes. Nature. 1978 May 18;273(5659):243–245. doi: 10.1038/273243a0. [DOI] [PubMed] [Google Scholar]
- Shporer M., Civan M. M. Nuclear magnetic resonance of sodium-23 linoleate-water. Basis for an alternative interpretation of sodium-23 spectra within cells. Biophys J. 1972 Jan;12(1):114–122. doi: 10.1016/S0006-3495(72)86074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
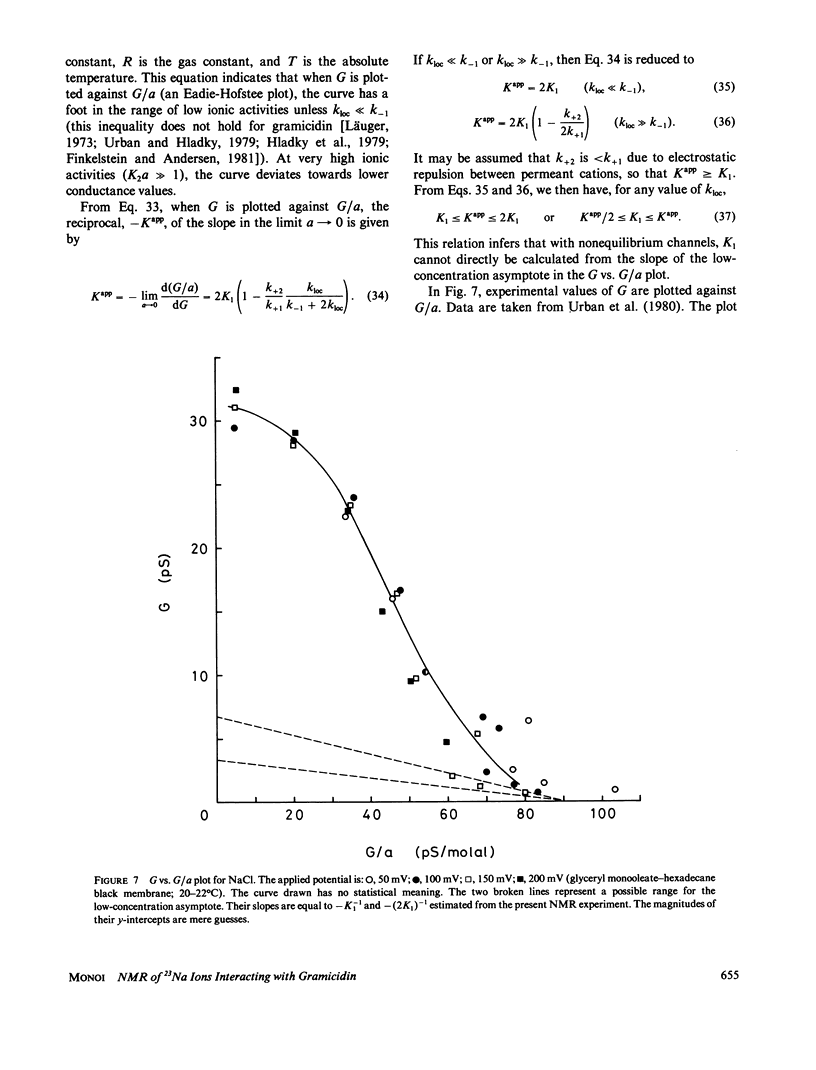
- Urban B. W., Hladky S. B., Haydon D. A. Ion movements in gramicidin pores. An example of single-file transport. Biochim Biophys Acta. 1980 Nov 4;602(2):331–354. doi: 10.1016/0005-2736(80)90316-8. [DOI] [PubMed] [Google Scholar]
- Urban B. W., Hladky S. B. Ion transport in the simplest single file pore. Biochim Biophys Acta. 1979 Jul 5;554(2):410–429. doi: 10.1016/0005-2736(79)90381-x. [DOI] [PubMed] [Google Scholar]
- Urry D. W., Goodall M. C., Glickson J. D., Mayers D. F. The gramicidin A transmembrane channel: characteristics of head-to-head dimerized (L,D) helices. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1907–1911. doi: 10.1073/pnas.68.8.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry D. W., Long M. M., Jacobs M., Harris R. D. Conformation and molecular mechanisms of carriers and channels. Ann N Y Acad Sci. 1975 Dec 30;264:203–220. doi: 10.1111/j.1749-6632.1975.tb31484.x. [DOI] [PubMed] [Google Scholar]
- Urry D. W., Venkatachalam C. M., Spisni A., Läuger P., Khaled M. A. Rate theory calculation of gramicidin single-channel currents using NMR-derived rate constants. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2028–2032. doi: 10.1073/pnas.77.4.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry D. W., Walker J. T., Trapane T. L. Ion interactions in (1-13C)D-Val8 and D-Leu14 analogs of gramicidin A, the helix sense of the channel and location of ion binding sites. J Membr Biol. 1982;69(3):225–231. doi: 10.1007/BF01870401. [DOI] [PubMed] [Google Scholar]
- Veatch W. R., Durkin J. T. Binding of thallium and other cations to the gramicidin A channel. Equilibrium dialysis study of gramicidin in phosphatidylcholine vesicles. J Mol Biol. 1980 Nov 15;143(4):411–417. doi: 10.1016/0022-2836(80)90220-x. [DOI] [PubMed] [Google Scholar]
- Veatch W. R., Fossel E. T., Blout E. R. The conformation of gramicidin A. Biochemistry. 1974 Dec 17;13(26):5249–5256. doi: 10.1021/bi00723a001. [DOI] [PubMed] [Google Scholar]
- Veatch W. R., Mathies R., Eisenberg M., Stryer L. Simultaneous fluorescence and conductance studies of planar bilayer membranes containing a highly active and fluorescent analog of gramicidin A. J Mol Biol. 1975 Nov 25;99(1):75–92. doi: 10.1016/s0022-2836(75)80160-4. [DOI] [PubMed] [Google Scholar]
- Veatch W., Stryer L. The dimeric nature of the gramicidin A transmembrane channel: conductance and fluorescence energy transfer studies of hybrid channels. J Mol Biol. 1977 Jun 15;113(1):89–102. doi: 10.1016/0022-2836(77)90042-0. [DOI] [PubMed] [Google Scholar]
- Wallace B. A., Veatch W. R., Blout E. R. Conformation of gramicidin A in phospholipid vesicles: circular dichroism studies of effects of ion binding, chemical modification, and lipid structure. Biochemistry. 1981 Sep 29;20(20):5754–5760. doi: 10.1021/bi00523a018. [DOI] [PubMed] [Google Scholar]
- Weinstein S., Wallace B. A., Blout E. R., Morrow J. S., Veatch W. Conformation of gramicidin A channel in phospholipid vesicles: a 13C and 19F nuclear magnetic resonance study. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4230–4234. doi: 10.1073/pnas.76.9.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein S., Wallace B. A., Morrow J. S., Veatch W. R. Conformation of the gramicidin A transmembrane channel: A 13C nuclear magnetic resonance study of 13C-enriched gramicidin in phosphatidylcholine vesicles. J Mol Biol. 1980 Oct 15;143(1):1–19. doi: 10.1016/0022-2836(80)90121-7. [DOI] [PubMed] [Google Scholar]


