Abstract
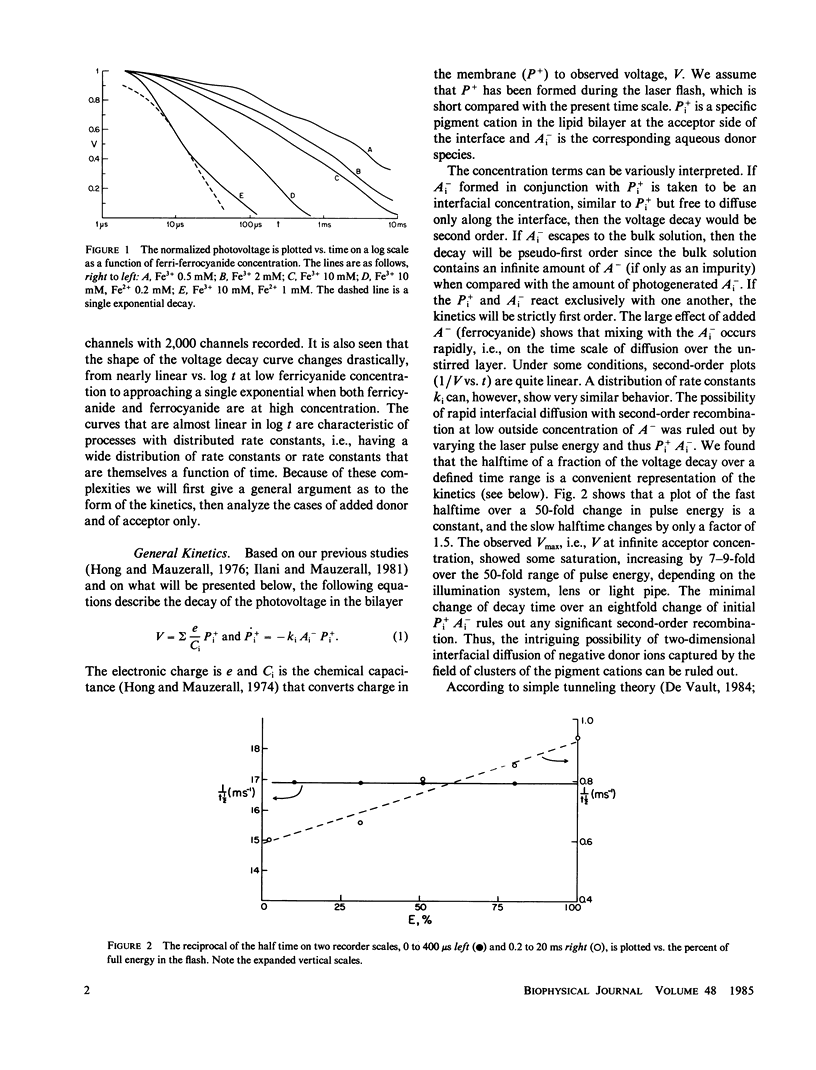
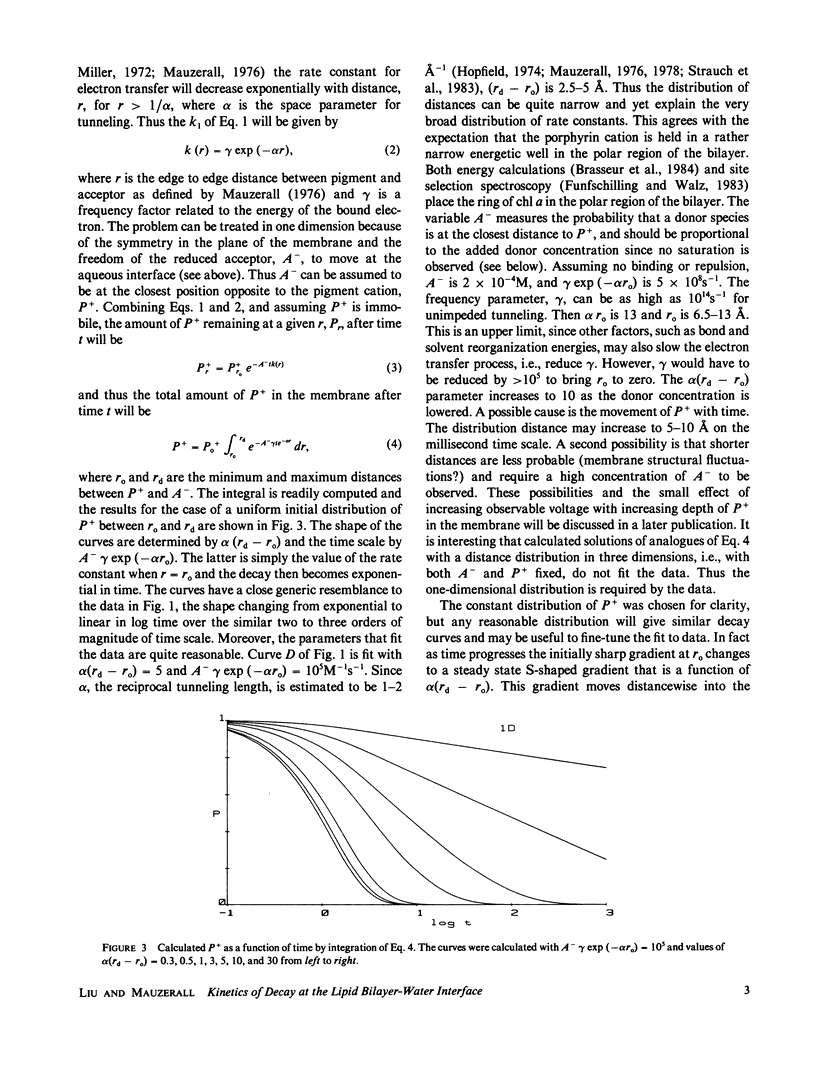
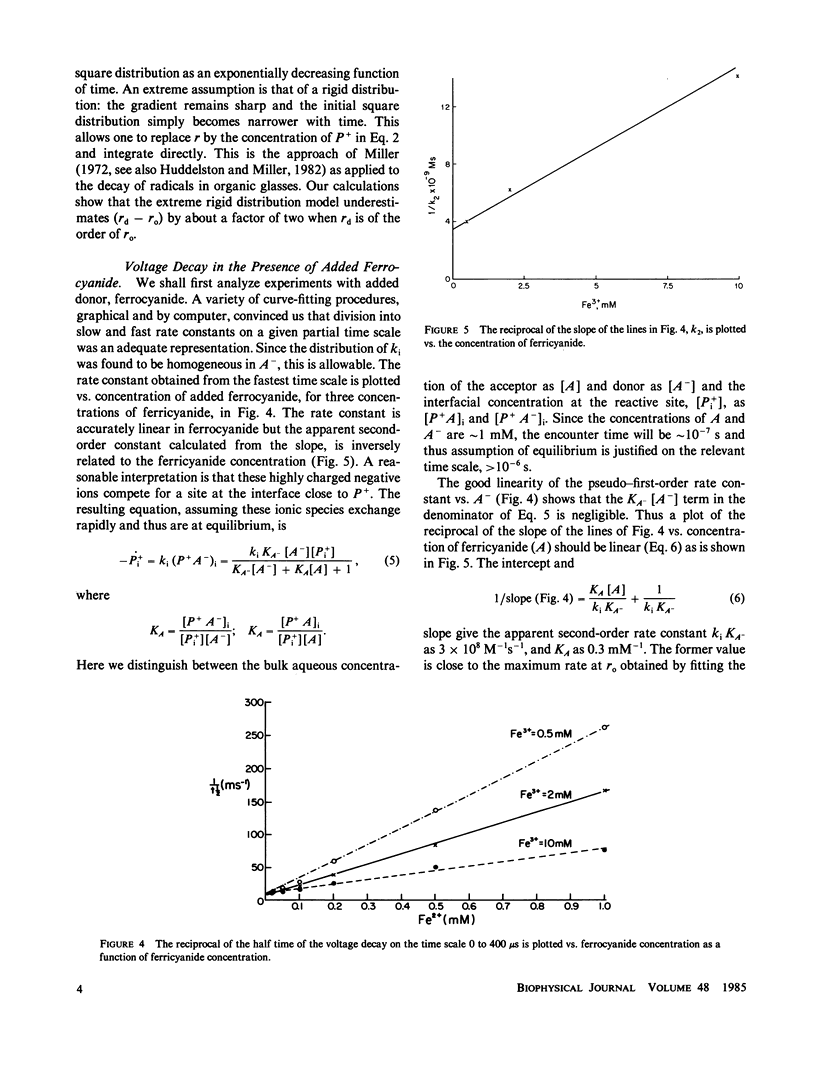
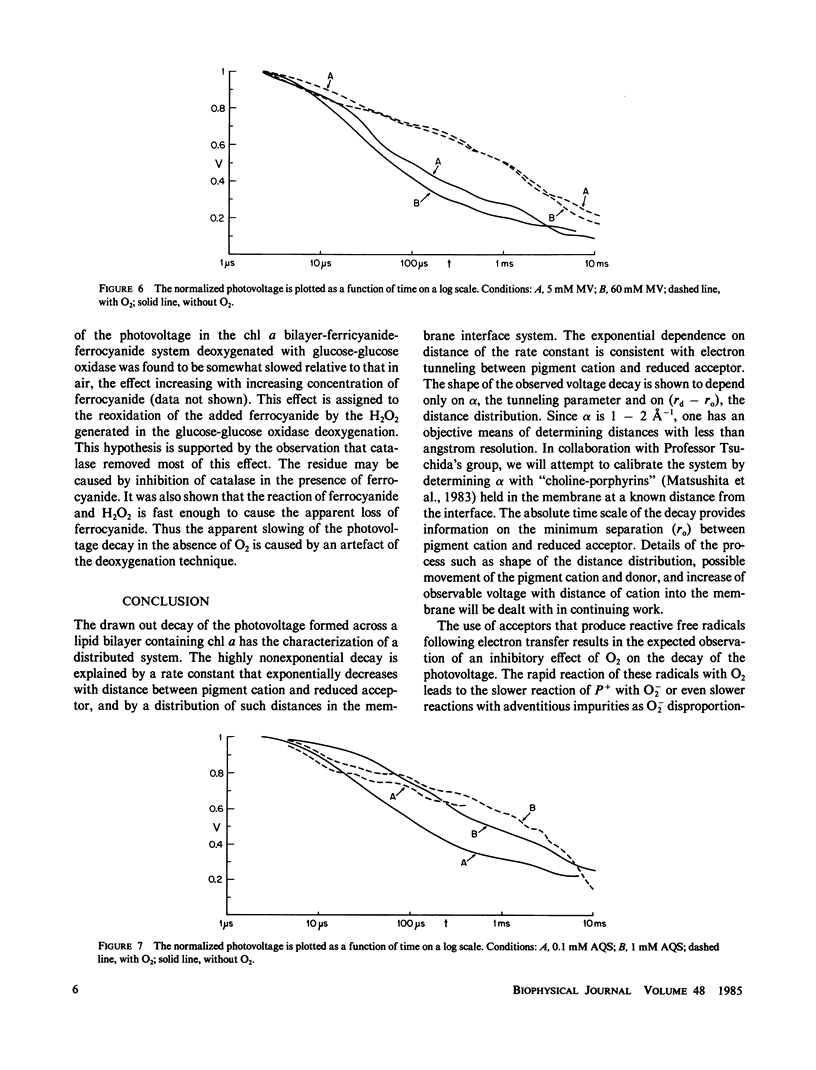
The decay kinetics of the photovoltage formed on pulsed illumination of a chlorophyll a- (chl a-) containing lecithin-bilayer adjacent to a ferricyanide solution on one side show characteristics of a system with distributed rate constants, i.e., the decay approaches linearity in log of time. The kinetics can be explained by a distribution of the chl cation over a few angstroms depth in the interfacial region of the bilayer and a rate constant exponentially dependent on distance as expected from tunneling theory. Addition of the donor ferrocyanide both increases the average rate and sharpens the distribution. There is a competitive inhibition by ferricyanide of the reaction of pigment cation with ferrocyanide. Removal of oxygen increases the rate of decay when an acceptor, methyl viologen or anthraquinone-2-sulfonate, forms oxygen-sensitive radicals. The cation charge does not cross the bilayer on a time scale of less than 0.01 s. These data define a reaction localized precisely in the finite interfacial region of the lipid bilayer-water interface.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hong F. T. Charge transfer across pigmented bilayer lipid membrane and its interfaces. Photochem Photobiol. 1976 Aug;24(2):155–189. doi: 10.1111/j.1751-1097.1976.tb06809.x. [DOI] [PubMed] [Google Scholar]
- Hong F. T., Mauzerall D. Interfacial photoreactions and chemical capacitance in lipid bilayers. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1564–1568. doi: 10.1073/pnas.71.4.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfield J. J. Electron transfer between biological molecules by thermally activated tunneling. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3640–3644. doi: 10.1073/pnas.71.9.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilani A., Liu T. M., Mauzerall D. The effect of oxygen on the amplitude of photodriven electron transfer across the lipid bilayer-water interface. Biophys J. 1985 May;47(5):679–684. doi: 10.1016/S0006-3495(85)83964-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilani A., Mauzerall D. The potential span of photoredox reactions of porphyrins and chlorophyll at the lipid bilayer-water interface. Biophys J. 1981 Jul;35(1):79–92. doi: 10.1016/S0006-3495(81)84775-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauzerall D. Electron transfer reactions and photoexcited porphyrins. Brookhaven Symp Biol. 1976 Jun 7;(28):64–73. [PubMed] [Google Scholar]
- Varnadore W. E., Jr, Arrieta R. T., Duchek J. R., Huebner J. S. Erythrosin and pH gradient induced photo-voltages in bilayer membranes. J Membr Biol. 1982;65(1-2):147–153. doi: 10.1007/BF01870478. [DOI] [PubMed] [Google Scholar]


