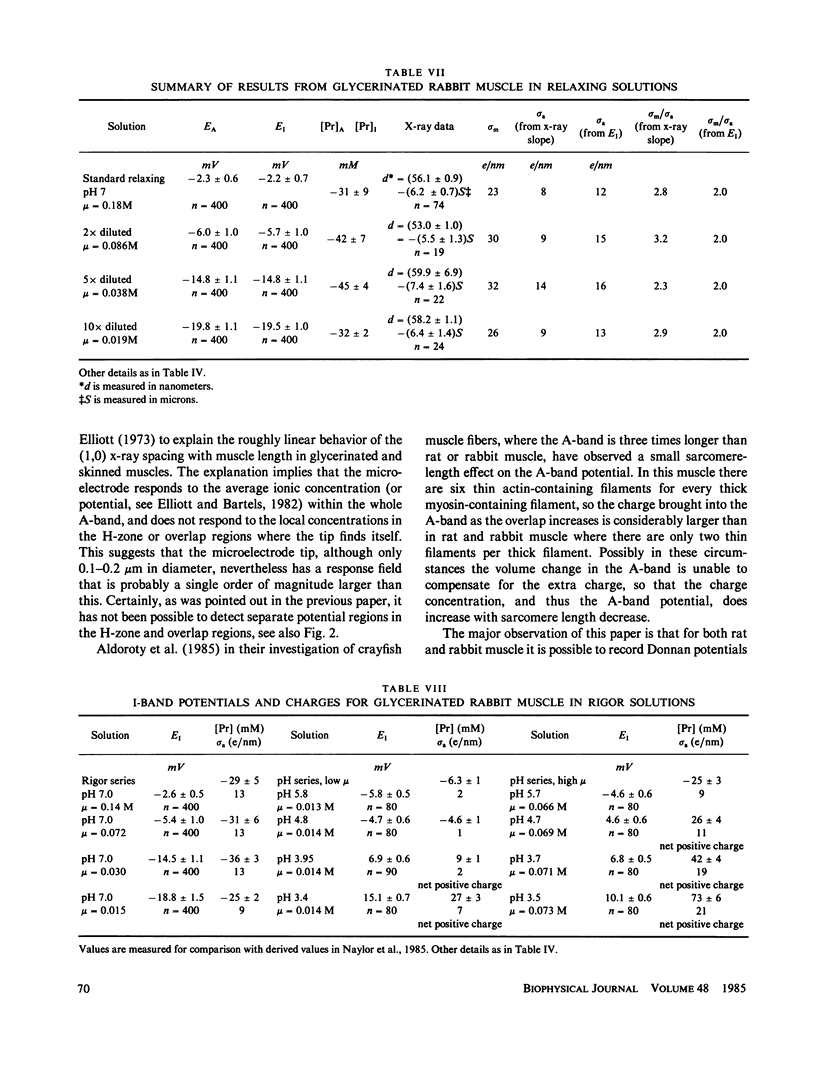
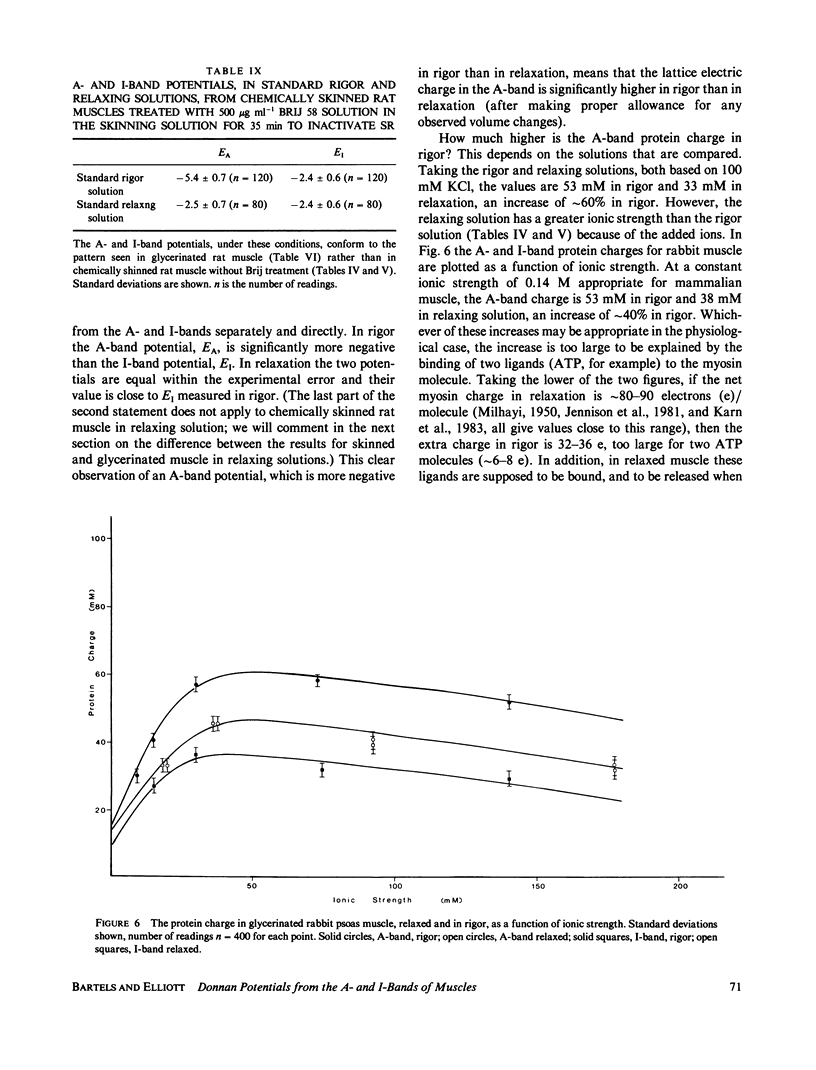
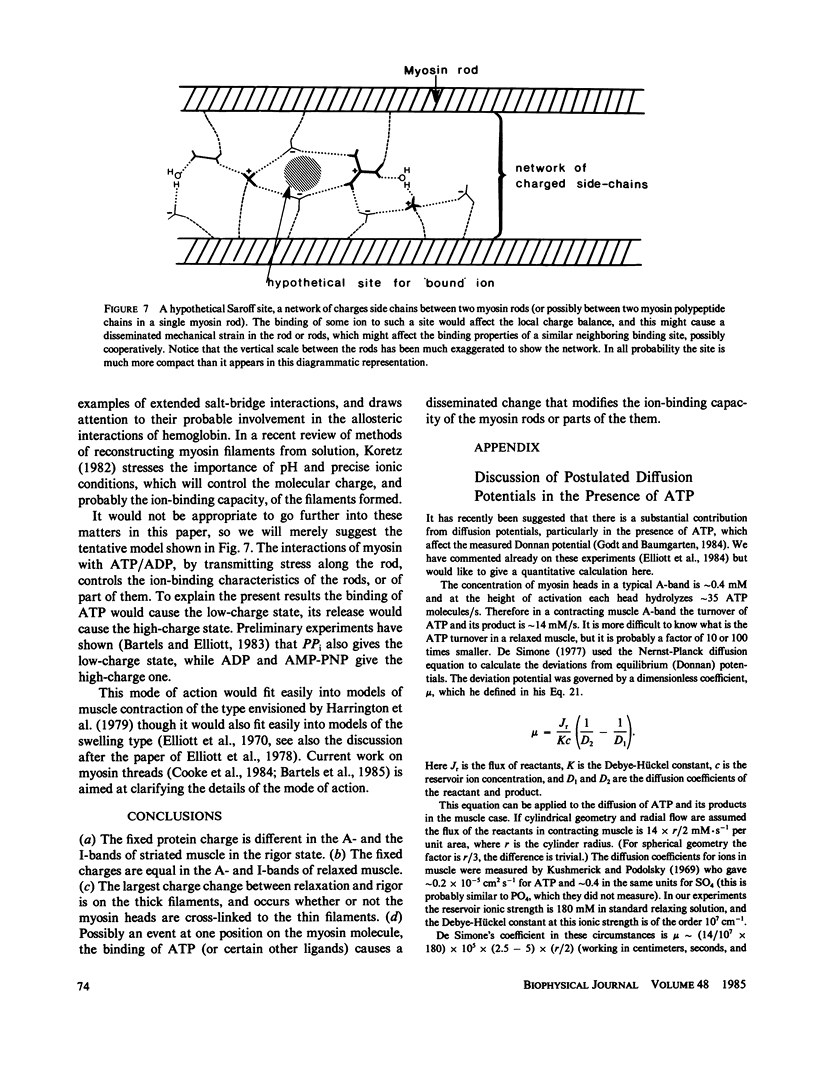
Abstract
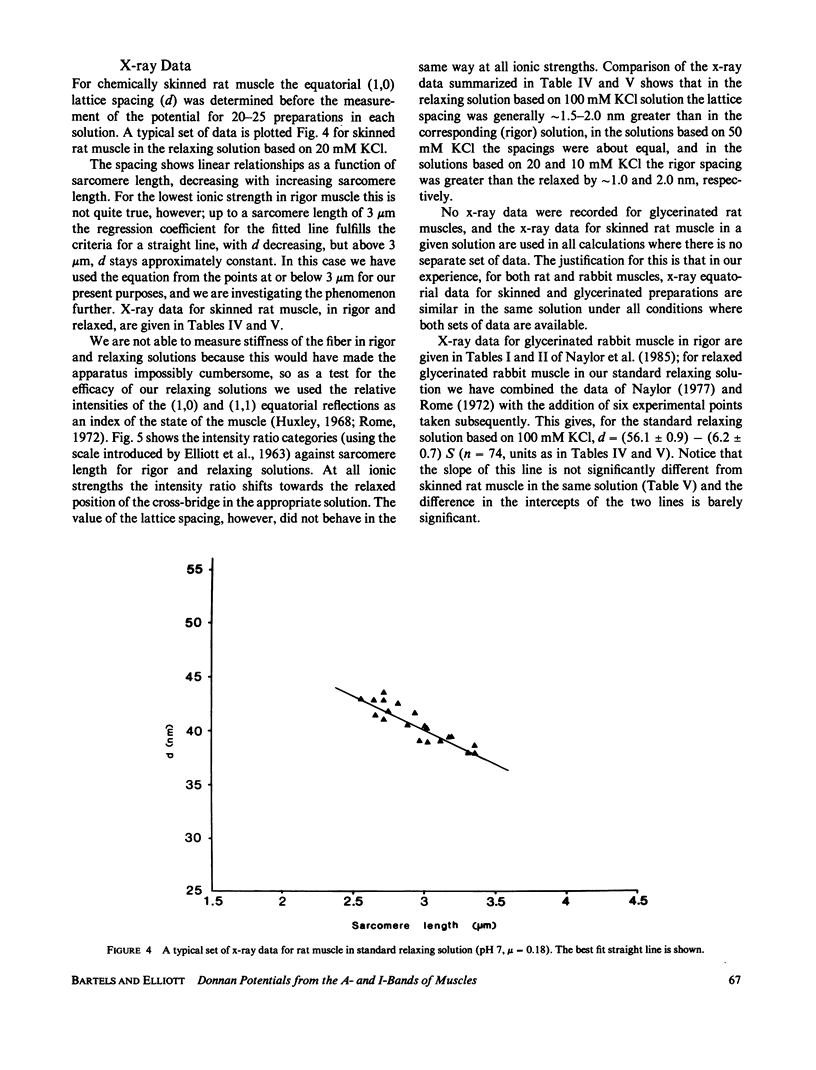
Using a combination of microelectrode measurements and high-power microscopy we have demonstrated that different Donnan potentials can be recorded from the A- and I-bands of glycerinated and chemically skinned muscles in rigor, so that the A-band fixed charge concentration exceeds the I-band fixed charge concentration in the rigor condition. In relaxation the two potentials, and therefore the two charge concentrations, are equal in the two bands. X-ray data are presented for relaxed and rigor rat semitendinosus muscle, chemically skinned, and actin and myosin filament charges are calculated under a variety of conditions. Our conclusions are that (a) the fixed (protein) charge is different in the A- and I-bands of striated muscle in the rigor state; (b) the fixed charges are equal in the A- and I-bands of relaxed muscle; (c) the largest charge change between relaxation and rigor is on the thick filament. This occurs whether or not the myosin heads are cross-linked to the thin filaments. (d) Possibly an event on the myosin molecule, the binding of ATP (or certain other ligands) causes a disseminated change that modifies the ion-binding capacity of the myosin rods, or part of them.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. J., Liljas A., Rossman M. G. Functional anion binding sites in dogfish M4 lactate dehydrogenase. J Mol Biol. 1973 Jun 5;76(4):519–528. doi: 10.1016/0022-2836(73)90489-0. [DOI] [PubMed] [Google Scholar]
- Aldoroty R. A., April E. W. Donnan potentials from striated muscle liquid crystals. A-band and I-band measurements. Biophys J. 1984 Dec;46(6):769–779. doi: 10.1016/S0006-3495(84)84075-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldoroty R. A., Garty N. B., April E. W. Donnan potentials from striated muscle liquid crystals. Sarcomere length dependence. Biophys J. 1985 Jan;47(1):89–95. doi: 10.1016/S0006-3495(85)83880-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels E. M., Skydsgaard J. M., Sten-Knudsen O. The time course of the latency relaxation as a function of the sarcomere length in frog and mammalian muscle. Acta Physiol Scand. 1979 Jun;106(2):129–137. doi: 10.1111/j.1748-1716.1979.tb06381.x. [DOI] [PubMed] [Google Scholar]
- DeSimone J. A. Perturbations in the structure of the double layer at an enzymic surface. J Theor Biol. 1977 Sep 21;68(2):225–240. doi: 10.1016/0022-5193(77)90161-8. [DOI] [PubMed] [Google Scholar]
- ELLIOTT G. F., WORTHINGTON C. R. A SMALL-ANGLE OPTICALLY FOCUSING X-RAY DIFFRACTION CAMERA IN BIOLOGICAL RESEARCH. I. J Ultrastruct Res. 1963 Aug;49:166–170. doi: 10.1016/s0022-5320(63)80044-1. [DOI] [PubMed] [Google Scholar]
- Elliott G. F., Bartels E. M., Cooke P. H., Jennison K. A reply to Godt and Baumgarten's potential and K+ activity in skinned muscle fibers: evidence for a simple Donnan equilibrium under physiological conditions. Biophys J. 1984 Feb;45(2):487–488. doi: 10.1016/S0006-3495(84)84173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott G. F., Bartels E. M. Donnan potential measurements in extended hexagonal polyelectrolyte gels such as muscle. Biophys J. 1982 May;38(2):195–199. doi: 10.1016/S0006-3495(82)84546-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott G. F. Donnan and osmotic effects in muscle fibres without membranes. J Mechanochem Cell Motil. 1973 May;2(1):83–89. [PubMed] [Google Scholar]
- Elliott G. F. Measurements of the electric charge and ion-binding of the protein filaments in intact muscle and cornea, with implications for filament assembly. Biophys J. 1980 Oct;32(1):95–97. doi: 10.1016/S0006-3495(80)84927-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott G. F., Rome E. M., Spencer M. A type of contraction hypothesis applicable to all muscles. Nature. 1970 May 2;226(5244):417–420. doi: 10.1038/226417a0. [DOI] [PubMed] [Google Scholar]
- Godt R. E., Baumgarten C. M. Potential and K+ activity in skinned muscle fibers. Evidence against a simple Donnan equilibrium. Biophys J. 1984 Feb;45(2):375–382. doi: 10.1016/S0006-3495(84)84161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J. Recent x-ray diffraction studies of muscle. Q Rev Biophys. 1968 Jun;1(2):177–216. doi: 10.1017/s0033583500000536. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. Structural difference between resting and rigor muscle; evidence from intensity changes in the lowangle equatorial x-ray diagram. J Mol Biol. 1968 Nov 14;37(3):507–520. doi: 10.1016/0022-2836(68)90118-6. [DOI] [PubMed] [Google Scholar]
- Kensler R. W., Stewart M. Frog skeletal muscle thick filaments are three-stranded. J Cell Biol. 1983 Jun;96(6):1797–1802. doi: 10.1083/jcb.96.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koretz J. F. Hybridization and reconstitution of thick-filament structure. Methods Enzymol. 1982;85(Pt B):20–55. doi: 10.1016/0076-6879(82)85008-8. [DOI] [PubMed] [Google Scholar]
- Kushmerick M. J., Podolsky R. J. Ionic mobility in muscle cells. Science. 1969 Dec 5;166(3910):1297–1298. doi: 10.1126/science.166.3910.1297. [DOI] [PubMed] [Google Scholar]
- LOEB G. I., SAROFF H. A. CHLORIDE- AND HYDROGEN-ION BINDING TO RIBONUCLEASE. Biochemistry. 1964 Dec;3:1819–1826. doi: 10.1021/bi00900a004. [DOI] [PubMed] [Google Scholar]
- Lowey S., Slayter H. S., Weeds A. G., Baker H. Substructure of the myosin molecule. I. Subfragments of myosin by enzymic degradation. J Mol Biol. 1969 May 28;42(1):1–29. doi: 10.1016/0022-2836(69)90483-5. [DOI] [PubMed] [Google Scholar]
- McLachlan A. D., Karn J. Periodic charge distributions in the myosin rod amino acid sequence match cross-bridge spacings in muscle. Nature. 1982 Sep 16;299(5880):226–231. doi: 10.1038/299226a0. [DOI] [PubMed] [Google Scholar]
- Mobley B. A., Eisenberg B. R. Sizes of components in frog skeletal muscle measured by methods of stereology. J Gen Physiol. 1975 Jul;66(1):31–45. doi: 10.1085/jgp.66.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor G. R. A simple circuit for automatic continuous recording of microelectrode resitance. Pflugers Arch. 1978 Dec 28;378(2):107–110. doi: 10.1007/BF00584442. [DOI] [PubMed] [Google Scholar]
- Naylor G. R. Average electrostatic potential between the filaments in striated muscle and its relation to a simple Donnan potential. Biophys J. 1982 May;38(2):201–204. doi: 10.1016/S0006-3495(82)84547-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor G. R., Bartels E. M., Bridgman T. D., Elliott G. F. Donnan potentials in rabbit psoas muscle in rigor. Biophys J. 1985 Jul;48(1):47–59. doi: 10.1016/S0006-3495(85)83759-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orentlicher M., Reuben J. P., Grundfest H., Brandt P. W. Calcium binding and tension development in detergent-treated muscle fibers. J Gen Physiol. 1974 Feb;63(2):168–186. doi: 10.1085/jgp.63.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemrick S. M., Edwards C. Differences in the charge distribution of glycerol-extracted muscle fibers in rigor, relaxation, and contraction. J Gen Physiol. 1974 Nov;64(5):551–567. doi: 10.1085/jgp.64.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz M. F. Electrostatic effects in proteins. Science. 1978 Sep 29;201(4362):1187–1191. doi: 10.1126/science.694508. [DOI] [PubMed] [Google Scholar]
- Poulsen F. R., Lowy J. Small-angle X-ray scattering from myosin heads in relaxed and rigor frog skeletal muscles. Nature. 1983 May 12;303(5913):146–152. doi: 10.1038/303146a0. [DOI] [PubMed] [Google Scholar]
- Reedy M. K., Holmes K. C., Tregear R. T. Induced changes in orientation of the cross-bridges of glycerinated insect flight muscle. Nature. 1965 Sep 18;207(5003):1276–1280. doi: 10.1038/2071276a0. [DOI] [PubMed] [Google Scholar]
- Rome E. Relaxation of glycerinated muscle: low-angle x-ray diffraction studies. J Mol Biol. 1972 Mar 28;65(2):331–345. doi: 10.1016/0022-2836(72)90285-9. [DOI] [PubMed] [Google Scholar]
- Scordilis S. P., Tedeschi H., Edwards C. Donnan potential of rabbit skeletal muscle myofibrils I: electrofluorochromometric detection of potential. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1325–1329. doi: 10.1073/pnas.72.4.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington C. R., Liu S. C. Structure of sarcoplasmic reticulum membranes at low resolution (17A). Arch Biochem Biophys. 1973 Aug;157(2):573–579. doi: 10.1016/0003-9861(73)90676-0. [DOI] [PubMed] [Google Scholar]



