Abstract
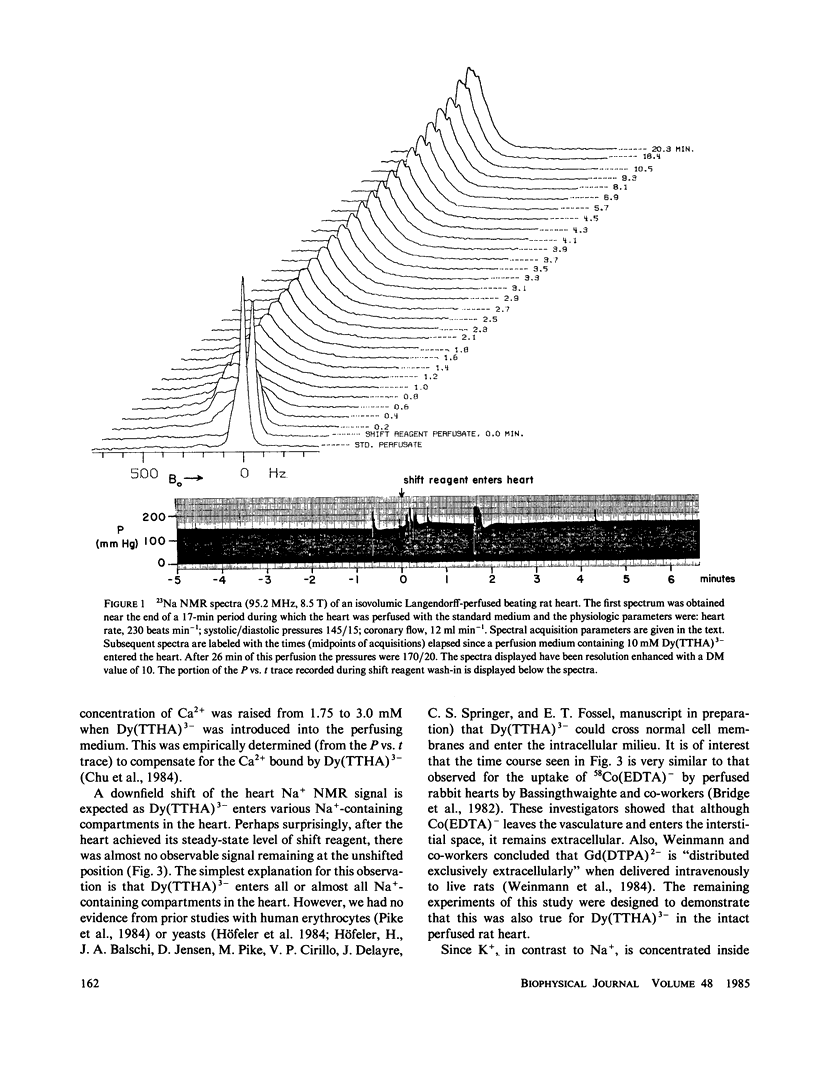
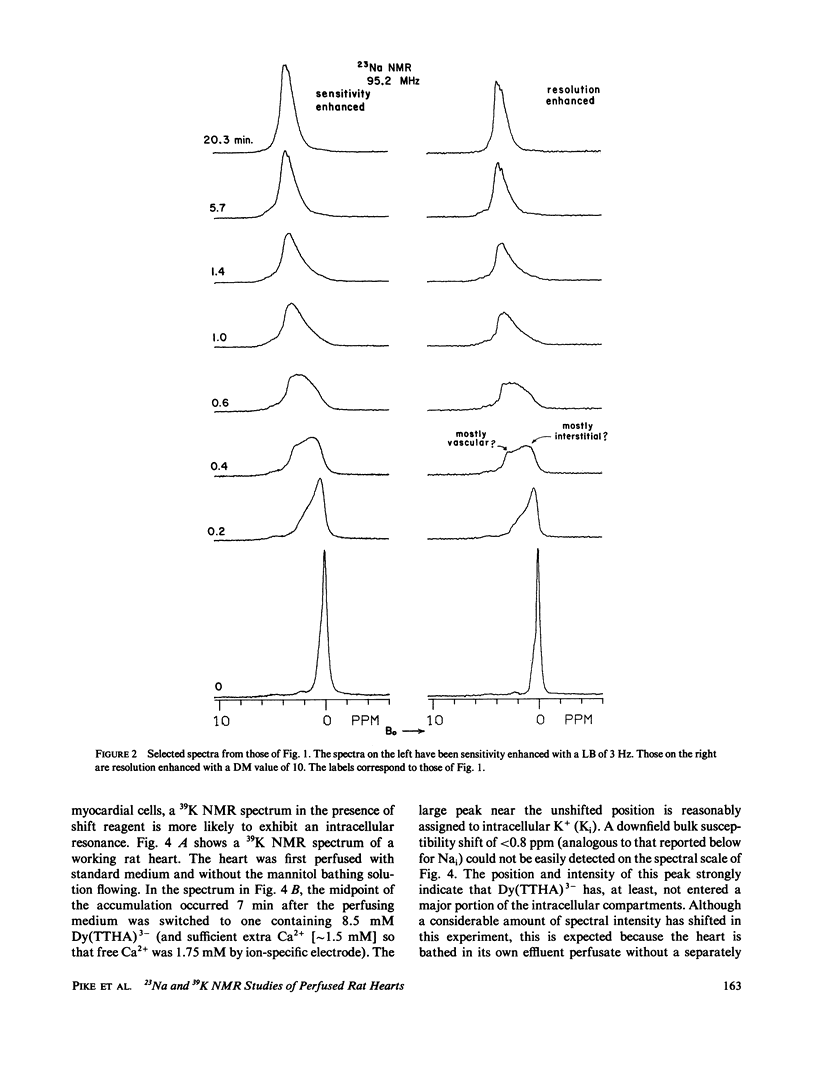
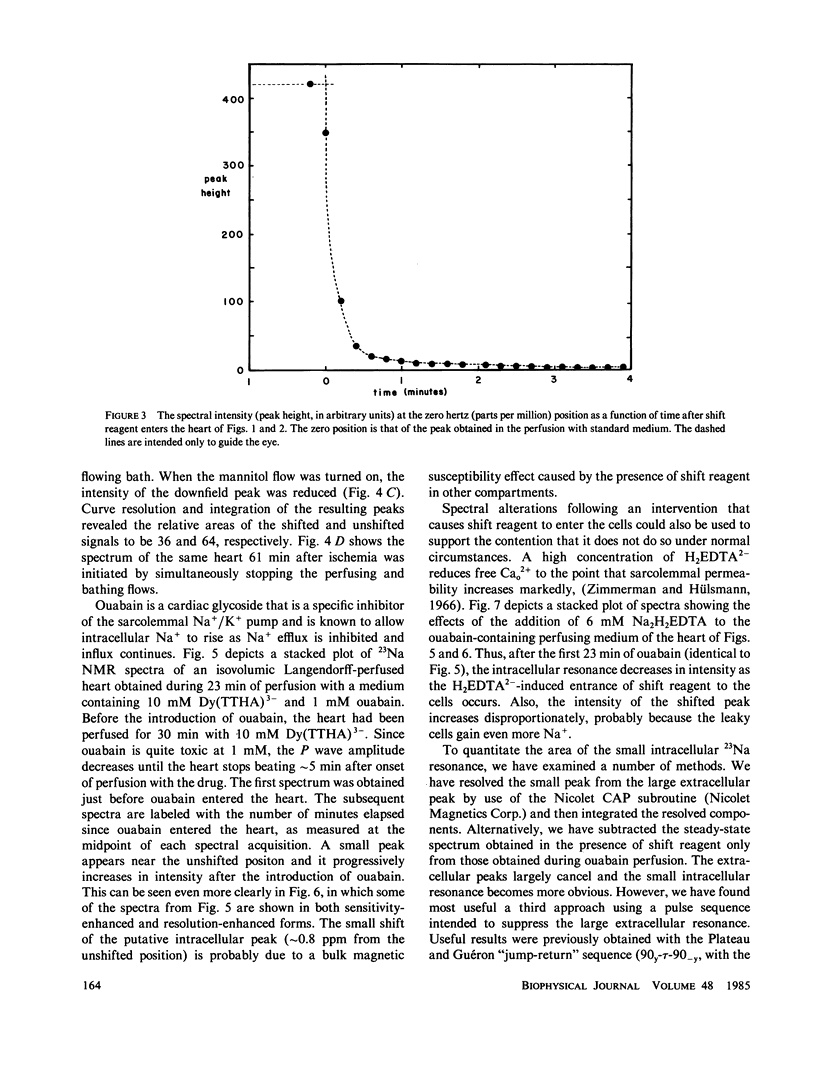
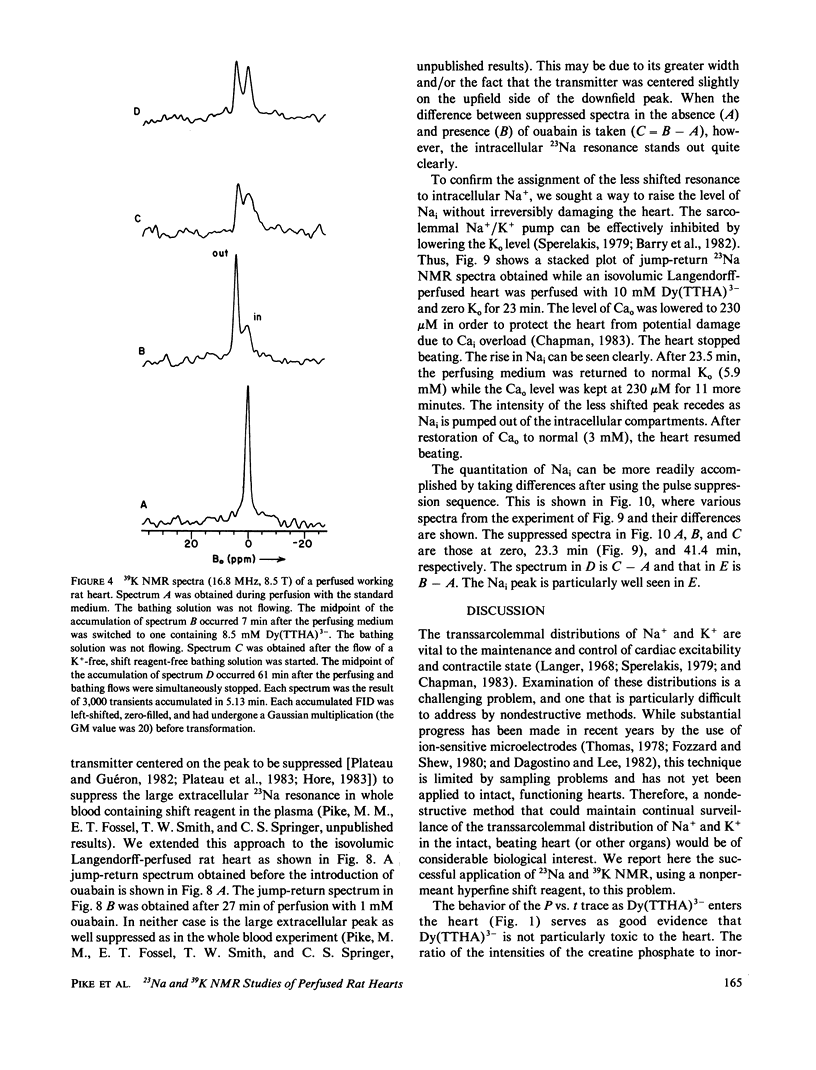
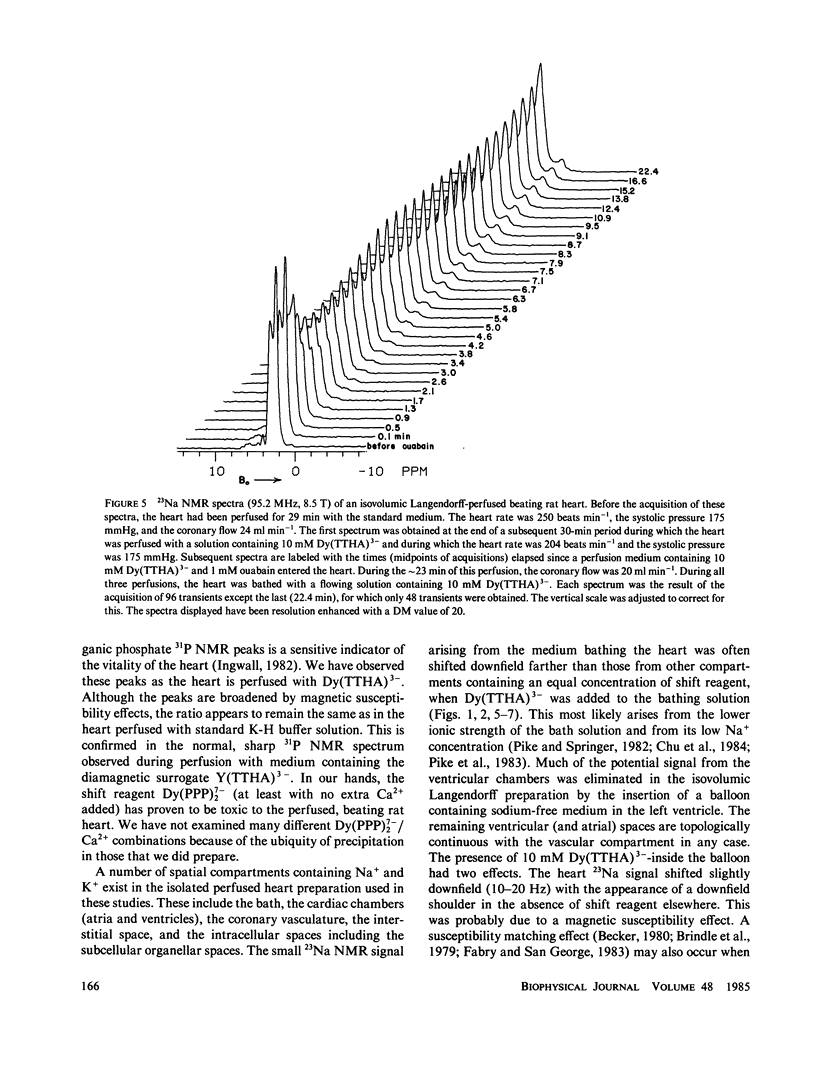
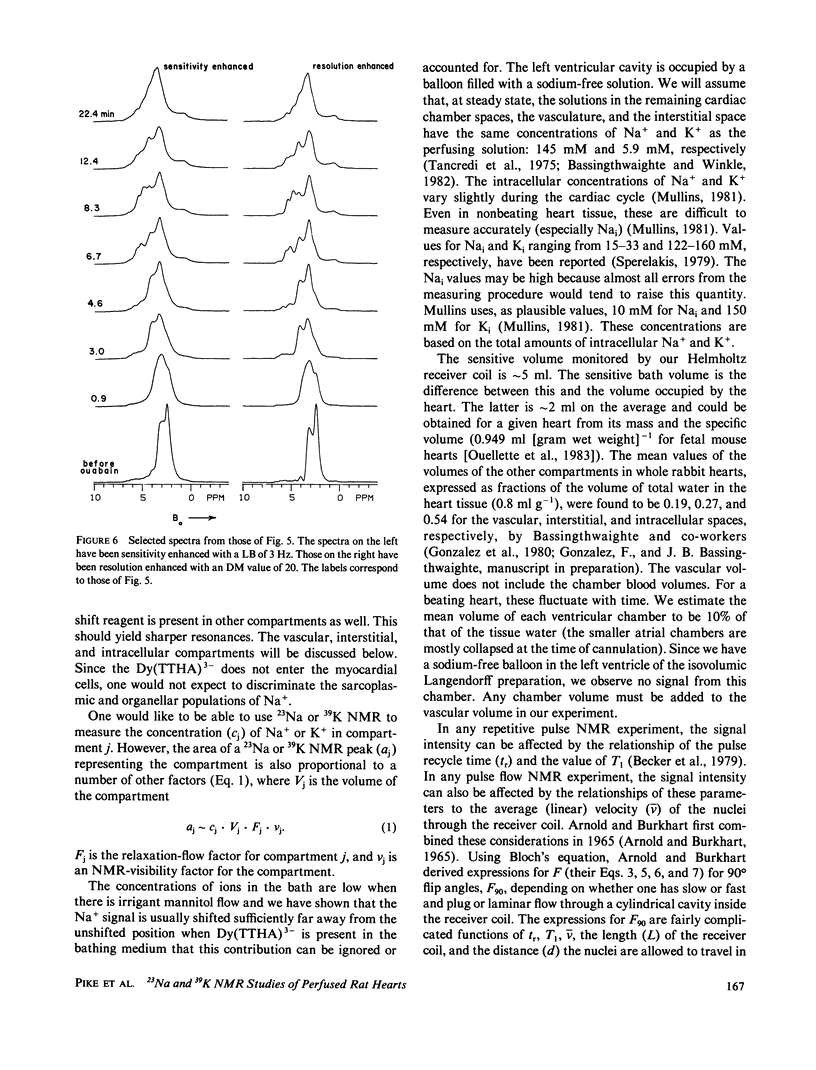
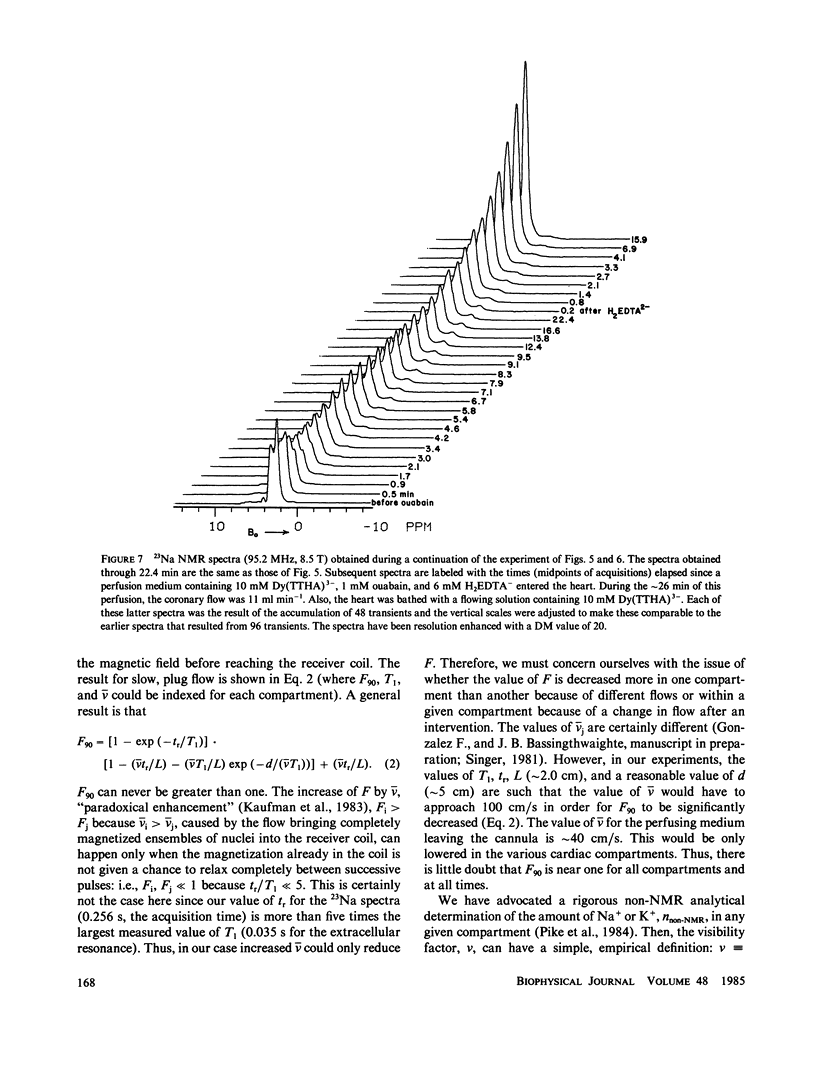
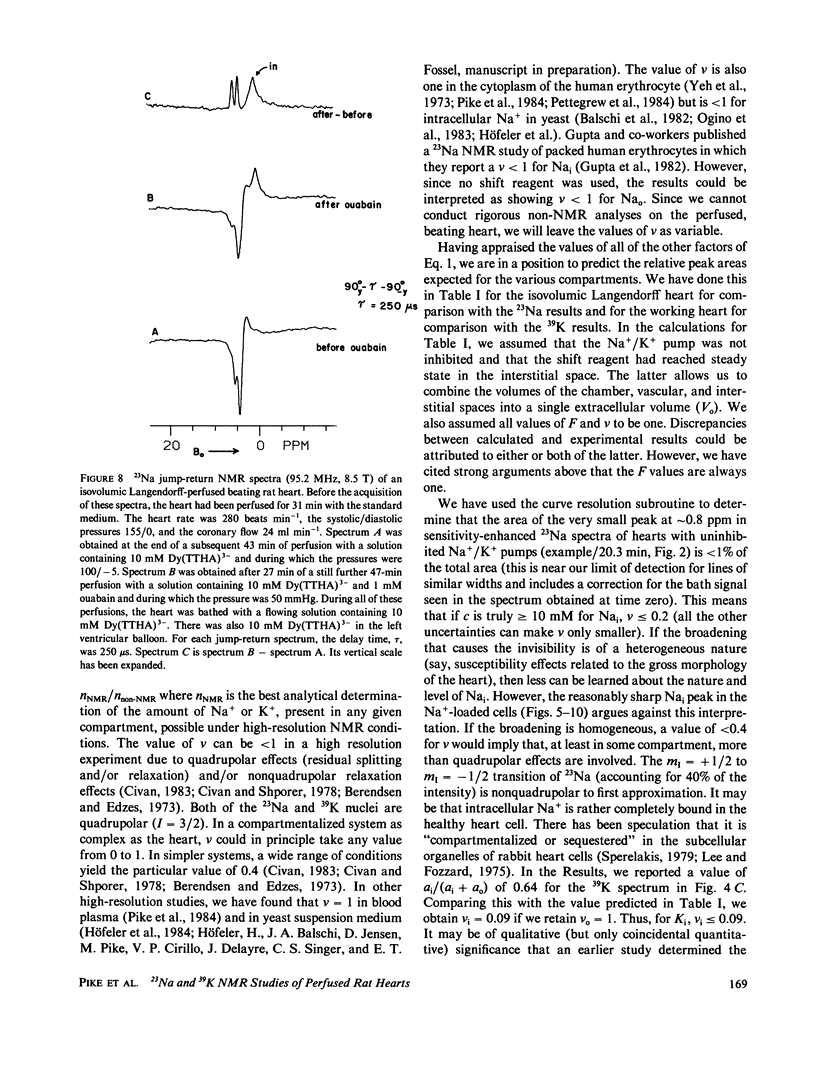
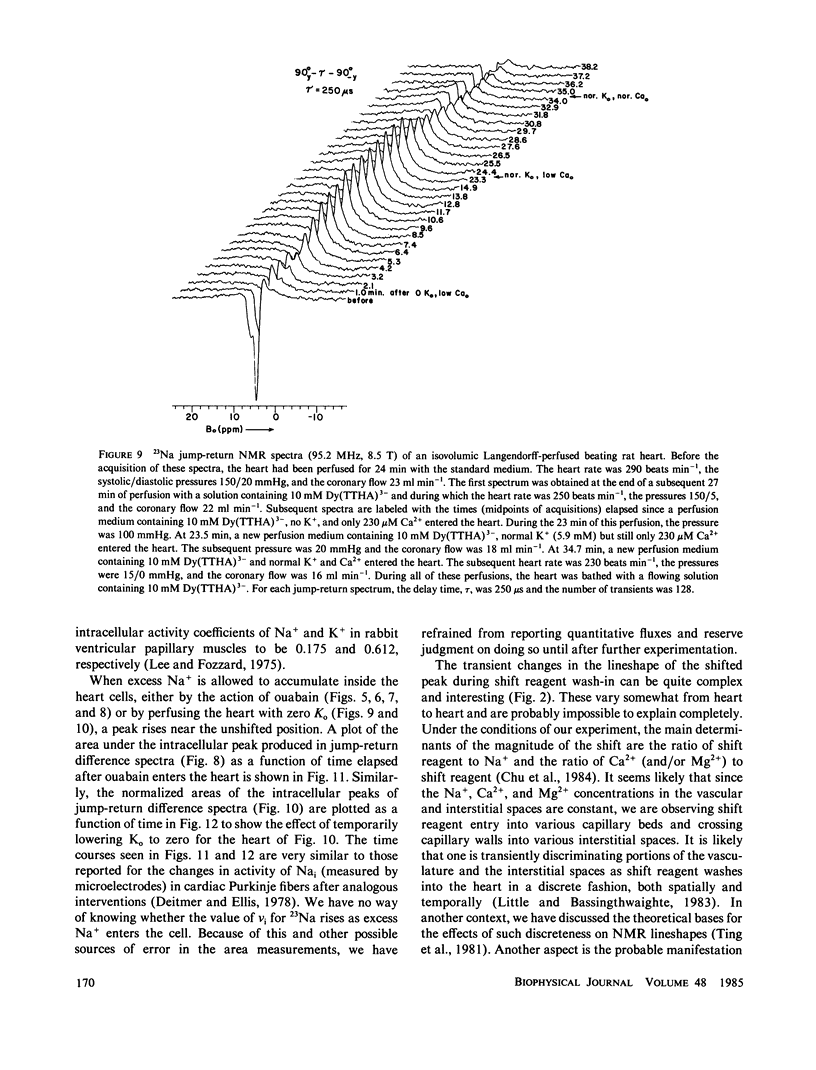
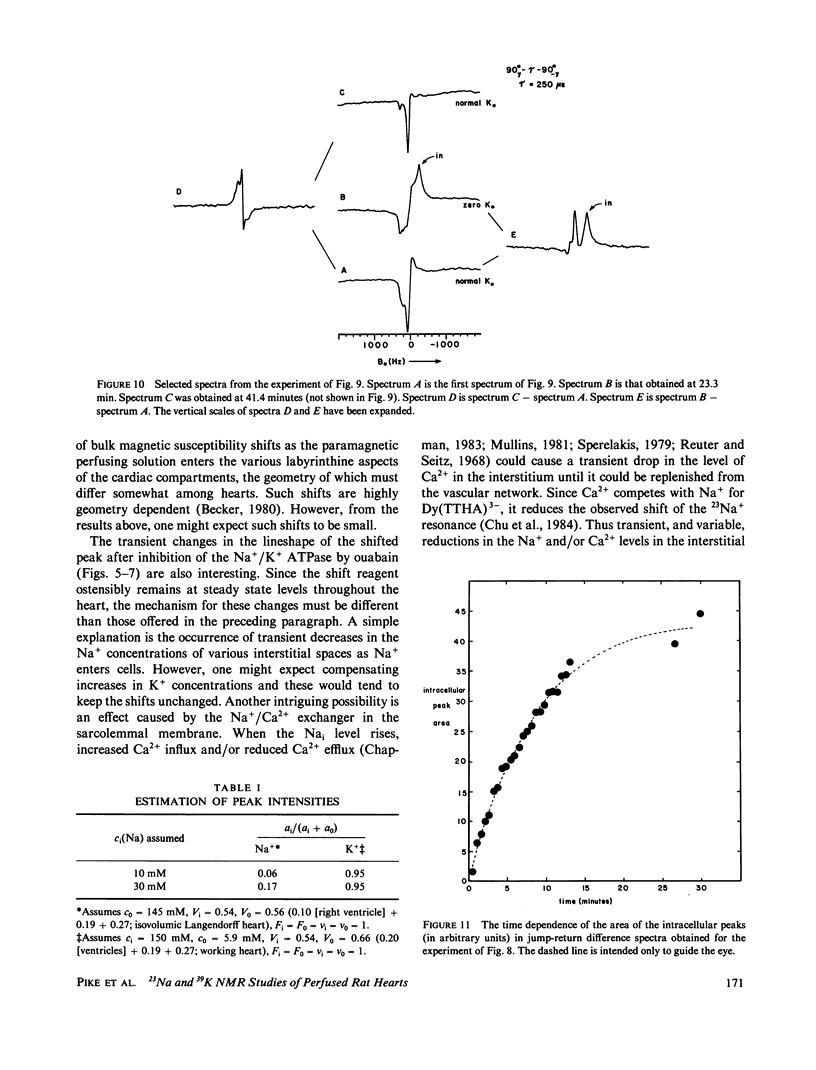
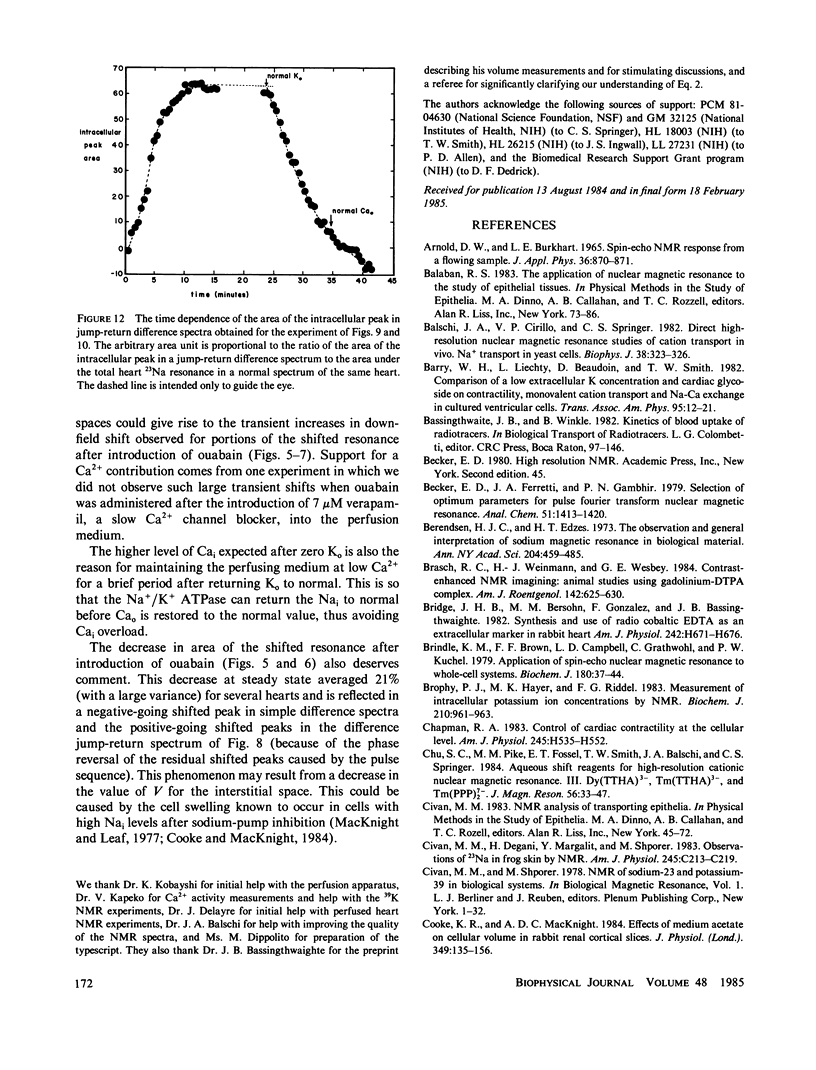
High-resolution 23Na and 39K nuclear magnetic resonance (NMR) spectra of perfused, beating rat hearts have been obtained in the absence and presence of the downfield shift reagent Dy(TTHA)3- in the perfusing medium. Evidence indicates that Dy(TTHA)3- enters essentially all extracellular spaces but does not enter intracellular spaces. It can thus be used to discriminate the resonances of the ions in these spaces. Experiments supporting this conclusion include interventions that inhibit the Na+/K+ pump such as the inclusion of ouabain in and the exclusion of K+ from the perfusing medium. In each of these experiments, a peak corresponding to intracellular sodium increased in intensity. In the latter experiment, the increase was reversed when the concentration of K+ in the perfusing medium was returned to normal. When the concentration of Ca2+ in the perfusing medium was also returned to normal, the previously quiescent heart resumed beating. In the beating heart where the Na+/K+ pump was not inhibited, the intensity of the intracellular Na+ resonance was less than 20% of that expected. Although the data are more sparse, the NMR visibility of the intracellular K+ signal appears to be no more than 20%.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balaban R. S. The application of nuclear magnetic resonance to the study of epithelial tissues. Prog Clin Biol Res. 1983;126:73–86. [PubMed] [Google Scholar]
- Balschi J. A., Cirillo V. P., Springer C. S., Jr Direct high-resolution nuclear magnetic resonance studies of cation transport in vivo, Na+ transport in yeast cells. Biophys J. 1982 Jun;38(3):323–326. doi: 10.1016/S0006-3495(82)84566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry W. H., Liechty L., Beaudoin D., Smith T. W. Comparison of effects of a low extracellular potassium concentration and cardiac glycoside on contractility, monovalent cation transport, and Na-Ca exchange in cultured ventricular cells. Trans Assoc Am Physicians. 1982;95:12–21. [PubMed] [Google Scholar]
- Berendsen H. J., Edzes H. T. The observation and general interpretation of sodium magnetic resonance in biological material. Ann N Y Acad Sci. 1973 Mar 30;204:459–485. doi: 10.1111/j.1749-6632.1973.tb30799.x. [DOI] [PubMed] [Google Scholar]
- Brasch R. C., Weinmann H. J., Wesbey G. E. Contrast-enhanced NMR imaging: animal studies using gadolinium-DTPA complex. AJR Am J Roentgenol. 1984 Mar;142(3):625–630. doi: 10.2214/ajr.142.3.625. [DOI] [PubMed] [Google Scholar]
- Bridge J. H., Bersohn M. M., Gonzalez F., Bassingthwaighte J. B. Synthesis and use of radio cobaltic EDTA as an extracellular marker in rabbit heart. Am J Physiol. 1982 Apr;242(4):H671–H676. doi: 10.1152/ajpheart.1982.242.4.H671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindle K. M., Brown F. F., Campbell I. D., Grathwohl C., Kuchel P. W. Application of spin-echo nuclear magnetic resonance to whole-cell systems. Membrane transport. Biochem J. 1979 Apr 15;180(1):37–44. doi: 10.1042/bj1800037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brophy P. J., Hayer M. K., Riddell F. G. Measurement of intracellular potassium ion concentrations by n.m.r. Biochem J. 1983 Mar 15;210(3):961–963. doi: 10.1042/bj2100961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. A. Control of cardiac contractility at the cellular level. Am J Physiol. 1983 Oct;245(4):H535–H552. doi: 10.1152/ajpheart.1983.245.4.H535. [DOI] [PubMed] [Google Scholar]
- Civan M. M., Degani H., Margalit Y., Shporer M. Observations of 23Na in frog skin by NMR. Am J Physiol. 1983 Sep;245(3):C213–C219. doi: 10.1152/ajpcell.1983.245.3.C213. [DOI] [PubMed] [Google Scholar]
- Cooke K. R., Macknight A. D. Effects of medium acetate on cellular volume in rabbit renal cortical slices. J Physiol. 1984 Apr;349:135–156. doi: 10.1113/jphysiol.1984.sp015148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagostino M., Lee C. O. Neutral carrier Na+- and Ca2+-selective microelectrodes for intracellular application. Biophys J. 1982 Dec;40(3):199–207. doi: 10.1016/S0006-3495(82)84475-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer J. W., Ellis D. The intracellular sodium activity of cardiac Purkinje fibres during inhibition and re-activation of the Na-K pump. J Physiol. 1978 Nov;284:241–259. doi: 10.1113/jphysiol.1978.sp012539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabry M. E., San George R. C. Effect of magnetic susceptibility on nuclear magnetic resonance signals arising from red cells: a warning. Biochemistry. 1983 Aug 16;22(17):4119–4125. doi: 10.1021/bi00286a020. [DOI] [PubMed] [Google Scholar]
- Fozzard H. A., Sheu S. S. Intracellular potassium and sodium activities of chick ventricular muscle during embryonic development. J Physiol. 1980 Sep;306:579–586. doi: 10.1113/jphysiol.1980.sp013416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingwall J. S. Phosphorus nuclear magnetic resonance spectroscopy of cardiac and skeletal muscles. Am J Physiol. 1982 May;242(5):H729–H744. doi: 10.1152/ajpheart.1982.242.5.H729. [DOI] [PubMed] [Google Scholar]
- Kaufman L., Crooks L., Sheldon P., Hricak H., Herfkens R., Bank W. The potential impact of nuclear magnetic resonance imaging on cardiovascular diagnosis. Circulation. 1983 Feb;67(2):251–257. doi: 10.1161/01.cir.67.2.251. [DOI] [PubMed] [Google Scholar]
- Langer G. A. Ion fluxes in cardiac excitation and contraction and their relation to myocardial contractility. Physiol Rev. 1968 Oct;48(4):708–757. doi: 10.1152/physrev.1968.48.4.708. [DOI] [PubMed] [Google Scholar]
- Lee C. O., Fozzard H. A. Activities of potassium and sodium ions in rabbit heart muscle. J Gen Physiol. 1975 Jun;65(6):695–708. doi: 10.1085/jgp.65.6.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S. E., Bassingthwaighte J. B. Plasma-soluble marker for intraorgan regional flows. Am J Physiol. 1983 Oct;245(4):H707–H712. doi: 10.1152/ajpheart.1983.245.4.H707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macknight A. D., Leaf A. Regulation of cellular volume. Physiol Rev. 1977 Jul;57(3):510–573. doi: 10.1152/physrev.1977.57.3.510. [DOI] [PubMed] [Google Scholar]
- Ogino T., den Hollander J. A., Shulman R. G. 39K, 23Na, and 31P NMR studies of ion transport in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5185–5189. doi: 10.1073/pnas.80.17.5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette A. J., Watson R. K., Billmire K., Dygert M. K., Ingwall J. S. Protein synthesis in the cultured fetal mouse heart: effects of deprivation of oxygen and oxidizable substrate. Biochemistry. 1983 Mar 1;22(5):1201–1207. doi: 10.1021/bi00274a033. [DOI] [PubMed] [Google Scholar]
- Pike M. M., Fossel E. T., Smith T. W., Springer C. S., Jr High-resolution 23Na-NMR studies of human erythrocytes: use of aqueous shift reagents. Am J Physiol. 1984 May;246(5 Pt 1):C528–C536. doi: 10.1152/ajpcell.1984.246.5.C528. [DOI] [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runge V. M., Clanton J. A., Lukehart C. M., Partain C. L., James A. E., Jr Paramagnetic agents for contrast-enhanced NMR imaging: a review. AJR Am J Roentgenol. 1983 Dec;141(6):1209–1215. doi: 10.2214/ajr.141.6.1209. [DOI] [PubMed] [Google Scholar]
- Tancredi R. G., Yipintsoi T., Bassingthwaighte J. B. Capillary and cell wall permeability to potassium in isolated dog hearts. Am J Physiol. 1975 Sep;229(3):537–544. doi: 10.1152/ajplegacy.1975.229.3.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting D. Z., Hagan P. S., Chan S. I., Doll J. D., Springer C. S., Jr Nuclear magnetic resonance studies of cation transport across vesicle bilayer membranes. Biophys J. 1981 May;34(2):189–216. doi: 10.1016/S0006-3495(81)84845-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmann H. J., Brasch R. C., Press W. R., Wesbey G. E. Characteristics of gadolinium-DTPA complex: a potential NMR contrast agent. AJR Am J Roentgenol. 1984 Mar;142(3):619–624. doi: 10.2214/ajr.142.3.619. [DOI] [PubMed] [Google Scholar]
- Yeh H. J., Brinley F. J., Jr, Becker E. D. Nuclear magnetic resonance studies on intracellular sodium in human erythrocytes and frog muscle. Biophys J. 1973 Jan;13(1):56–71. doi: 10.1016/S0006-3495(73)85969-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman A. N., Hülsmann W. C. Paradoxical influence of calcium ions on the permeability of the cell membranes of the isolated rat heart. Nature. 1966 Aug 6;211(5049):646–647. doi: 10.1038/211646a0. [DOI] [PubMed] [Google Scholar]


