Abstract
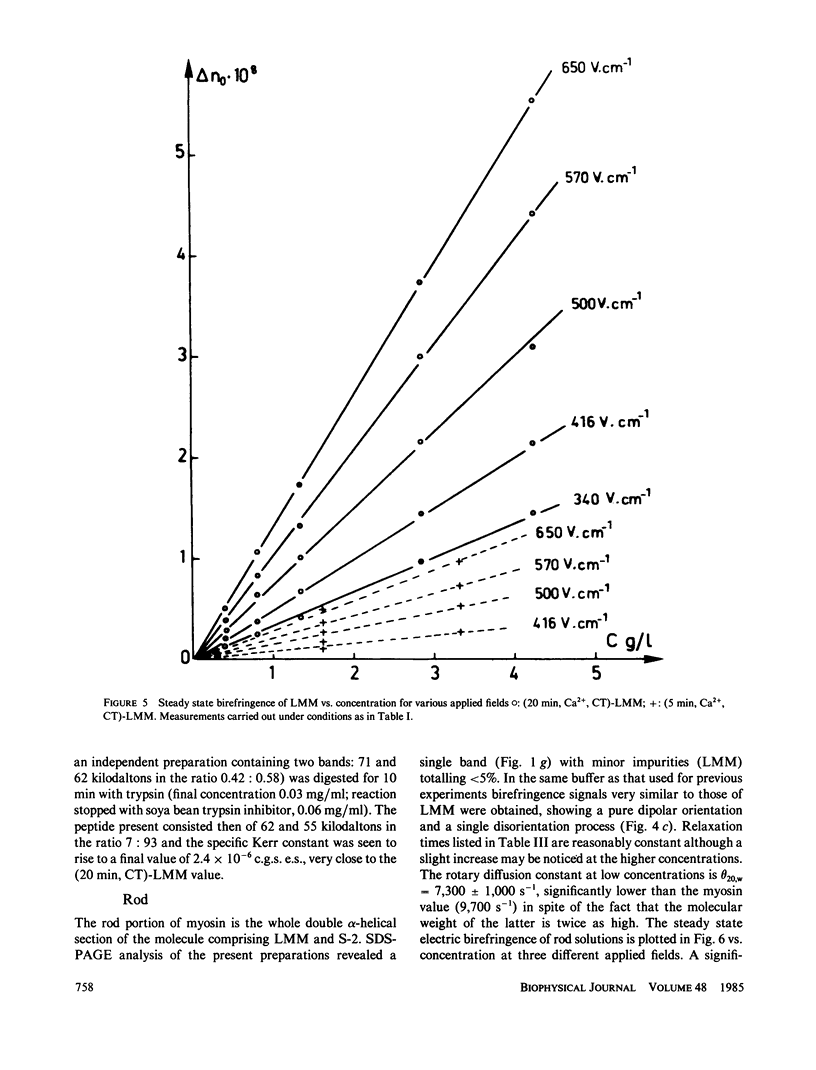
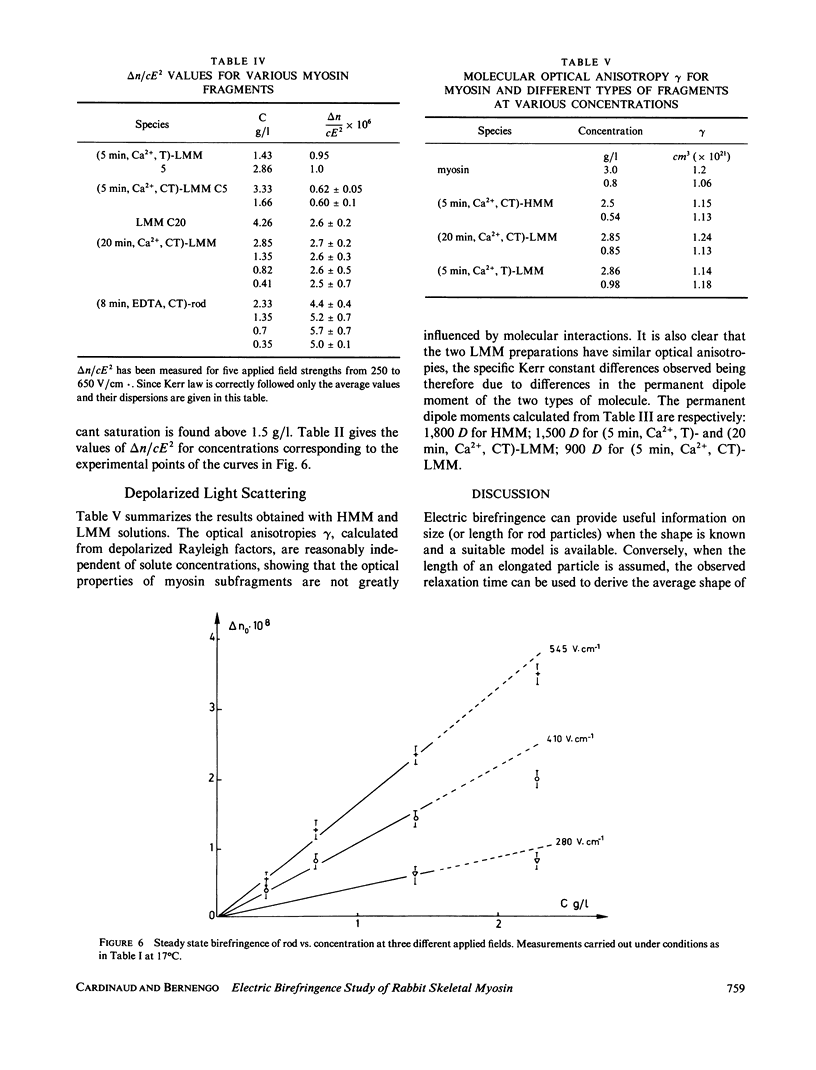
Electric birefringence measurements and depolarized light scattering experiments were performed with HMM, LMM, and rod, the three fragments of myosin, under conditions (0.3 M KCl, 0.02 M PO4, pH 7.3) the medium currently used for biochemical assays of myosin in its native state as well as of its subfragments. The comparison of myosin and rod relaxation times (17.2 and 22.8 microseconds, respectively) suggests that the average bend angle in the tail is sharper in intact myosin (90 degrees) whereas rod, when detached from the heads, is a more elongated species with an average bend angle of 120-135 degrees. The LMM relaxation time (6.4 microseconds) is consistent with a rigid linear stick model of length 78 nm. Flexibility in myosin tail is thus confirmed as located in the HMM-LMM hinge. LMM and rod did not exhibit any significant variation of their apparent relaxation times with concentration and the decay curves were best fitted by a single exponential, evidence that the concentration of parallel staggered dimers was negligible in the concentration range studied here (0-7 g/l). This observation lends support to previous results obtained with myosin. Respective HMM, LMM, and rod molecular weights and homogeneity as evaluated by SDS-PAGE analysis were correlated to the Kerr constants of their solutions. Large variations in LMM Kerr constants could be related to the loss of a COOH-terminal peptide on prolonged chymotryptic digestion. Electric birefringence combined with depolarized light scattering is presented as a potential method for net charge distribution studies.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akutagawa T., Ooi T. Fragments responsible for the low solubility of light meromyosin obtained by limited proteolysis. J Biochem. 1982 Oct;92(4):999–1007. doi: 10.1093/oxfordjournals.jbchem.a134056. [DOI] [PubMed] [Google Scholar]
- Bernengo J. C., Cardinaud R. State of myosin in solution. Electric birefringence and dynamic light-scattering studies. J Mol Biol. 1982 Aug 15;159(3):501–517. doi: 10.1016/0022-2836(82)90298-4. [DOI] [PubMed] [Google Scholar]
- Bernengo J. C., Roux B., Hanss M. Electrical birefringence apparatus for conducting solutions. Rev Sci Instrum. 1973 Aug;44(8):1083–1086. doi: 10.1063/1.1686306. [DOI] [PubMed] [Google Scholar]
- Bernengo J. C., Roux B., Herbage D. Electrical birefringence study of monodisperse collagen solutions. Biopolymers. 1974;13(3):641–647. doi: 10.1002/bip.1974.360130315. [DOI] [PubMed] [Google Scholar]
- Bálint M., Szilágyi L., Fekete G., Blazsó M., Biró N. A. Studies on proteins and protein complexes of muscle by means of proteolysis. V. Fragmentation of light meromyosin by trypsin. J Mol Biol. 1968 Oct 28;37(2):317–330. doi: 10.1016/0022-2836(68)90271-4. [DOI] [PubMed] [Google Scholar]
- Cardinaud R., Dassin E., Pelletier F. Heterogeneity of subfragment-1 preparations from myofibril digestion by trypsin. Biochem Biophys Res Commun. 1973 Jun 8;52(3):1057–1063. doi: 10.1016/0006-291x(73)91045-0. [DOI] [PubMed] [Google Scholar]
- Cardinaud R., Drifford M. Quasi-elastic light scattering studies of rabbit skeletal myosin solutions. J Muscle Res Cell Motil. 1982 Sep;3(3):313–332. doi: 10.1007/BF00713040. [DOI] [PubMed] [Google Scholar]
- Cardinaud R. Fate of the light chains in the course of proteolytic digestion of rabbit fast skeletal myosin. Biochimie. 1980;62(2-3):135–145. doi: 10.1016/s0300-9084(80)80189-1. [DOI] [PubMed] [Google Scholar]
- Cardinaud R. Proteolytic fragmentation of myosin: location of SH-1 and SH-2 thiols. Biochimie. 1979;61(7):807–821. doi: 10.1016/s0300-9084(79)80275-8. [DOI] [PubMed] [Google Scholar]
- Craig R., Smith R., Kendrick-Jones J. Light-chain phosphorylation controls the conformation of vertebrate non-muscle and smooth muscle myosin molecules. 1983 Mar 31-Apr 6Nature. 302(5907):436–439. doi: 10.1038/302436a0. [DOI] [PubMed] [Google Scholar]
- Elliott A., Offer G. Shape and flexibility of the myosin molecule. J Mol Biol. 1978 Aug 25;123(4):505–519. doi: 10.1016/0022-2836(78)90204-8. [DOI] [PubMed] [Google Scholar]
- Frank G., Weeds A. G. The amino-acid sequence of the alkali light chains of rabbit skeletal-muscle myosin. Eur J Biochem. 1974 May 15;44(2):317–334. doi: 10.1111/j.1432-1033.1974.tb03489.x. [DOI] [PubMed] [Google Scholar]
- García de la Torre J., Bloomfield V. A. Conformation of myosin in dilute solution as estimated from hydrodynamic properties. Biochemistry. 1980 Oct 28;19(22):5118–5123. doi: 10.1021/bi00563a028. [DOI] [PubMed] [Google Scholar]
- Godfrey J. E., Harrington W. F. Self-association in the myosin system at high ionic strength. I. Sensitivity of the interaction to pH and ionic environment. Biochemistry. 1970 Feb 17;9(4):886–893. doi: 10.1021/bi00806a025. [DOI] [PubMed] [Google Scholar]
- Harrington W. F., Burke M. Geometry of the myosin dimer in high-salt media. I. Association behavior of rod segments from myosin. Biochemistry. 1972 Apr 11;11(8):1448–1455. doi: 10.1021/bi00758a019. [DOI] [PubMed] [Google Scholar]
- Harrison R. G., Lowey S., Cohen C. Assembly of myosin. J Mol Biol. 1971 Aug 14;59(3):531–535. doi: 10.1016/0022-2836(71)90317-2. [DOI] [PubMed] [Google Scholar]
- Harvey S. C., Cheung H. C. Fluorescence depolarization studies on the flexibility of myosin rod. Biochemistry. 1977 Nov 29;16(24):5181–5187. doi: 10.1021/bi00643a004. [DOI] [PubMed] [Google Scholar]
- Highsmith S., Jardetzky O. Internal motions in myosin. 2. Biochemistry. 1981 Feb 17;20(4):780–783. doi: 10.1021/bi00507a020. [DOI] [PubMed] [Google Scholar]
- Highsmith S., Kretzschmar K. M., O'Konski C. T., Morales M. F. Flexibility of myosin rod, light meromyosin, and myosin subfragment-2 in solution. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4986–4990. doi: 10.1073/pnas.74.11.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highsmith S. The dynamics of myosin and actin in solution are compatible with the mechanical features of the cross-bridge hypothesis. Biochim Biophys Acta. 1981 Nov 9;639(1):31–39. doi: 10.1016/0304-4173(81)90003-3. [DOI] [PubMed] [Google Scholar]
- Highsmith S., Wang C. C., Zero K., Pecora R., Jardetzky O. Bending motions and internal motions in myosin rod. Biochemistry. 1982 Mar 16;21(6):1192–1197. doi: 10.1021/bi00535a013. [DOI] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Hvidt S., Nestler F. H., Greaser M. L., Ferry J. D. Flexibility of myosin rod determined from dilute solution viscoelastic measurements. Biochemistry. 1982 Aug 17;21(17):4064–4073. doi: 10.1021/bi00260a024. [DOI] [PubMed] [Google Scholar]
- Kobayasi S., Totsuka T. Electric birefringence of myosin subfragments. Biochim Biophys Acta. 1975 Feb 17;376(2):375–385. doi: 10.1016/0005-2728(75)90029-8. [DOI] [PubMed] [Google Scholar]
- Lowey S., Slayter H. S., Weeds A. G., Baker H. Substructure of the myosin molecule. I. Subfragments of myosin by enzymic degradation. J Mol Biol. 1969 May 28;42(1):1–29. doi: 10.1016/0022-2836(69)90483-5. [DOI] [PubMed] [Google Scholar]
- Margossian S. S., Stafford W. F., 3rd, Lowey S. Homogeneity of myosin subfragments by equilibrium centrifugation. Biochemistry. 1981 Apr 14;20(8):2151–2155. doi: 10.1021/bi00511a012. [DOI] [PubMed] [Google Scholar]
- Marion C., Bezot P., Hesse-Bezot C., Roux B., Bernengo J. C. Conformation of chromatin oligomers. A new argument for a change with the hexanucleosome. Eur J Biochem. 1981 Nov;120(1):169–176. doi: 10.1111/j.1432-1033.1981.tb05685.x. [DOI] [PubMed] [Google Scholar]
- McLachlan A. D., Karn J. Periodic charge distributions in the myosin rod amino acid sequence match cross-bridge spacings in muscle. Nature. 1982 Sep 16;299(5880):226–231. doi: 10.1038/299226a0. [DOI] [PubMed] [Google Scholar]
- Miyahara M., Noda H. Electric and flow birefringence properties of myosin in aqueous urea and sodium pyrophosphate. J Biochem. 1979 Jul;86(1):239–248. [PubMed] [Google Scholar]
- Montague C., Carlson F. D. Preparation of contractile proteins for photon correlation spectroscopic and classical light-scattering studies. Methods Enzymol. 1982;85(Pt B):562–570. doi: 10.1016/0076-6879(82)85051-9. [DOI] [PubMed] [Google Scholar]
- Nyitray L., Mocz G., Szilagyi L., Balint M., Lu R. C., Wong A., Gergely J. The proteolytic substructure of light meromyosin. Localization of a region responsible for the low ionic strength insolubility of myosin. J Biol Chem. 1983 Nov 10;258(21):13213–13220. [PubMed] [Google Scholar]
- Parry D. A. Structure of rabbit skeletal myosin. Analysis of the amino acid sequences of two fragments from the rod region. J Mol Biol. 1981 Dec 5;153(2):459–464. doi: 10.1016/0022-2836(81)90290-4. [DOI] [PubMed] [Google Scholar]
- Sutoh K., Sutoh K., Karr T., Harrington W. F. Isolation and physico-chemical properties of a high molecular weight subfragment-2 of myosin. J Mol Biol. 1978 Nov 25;126(1):1–22. doi: 10.1016/0022-2836(78)90276-0. [DOI] [PubMed] [Google Scholar]
- Takahashi K. Topography of the myosin molecule as visualized by an improved negative staining method. J Biochem. 1978 Mar;83(3):905–908. doi: 10.1093/oxfordjournals.jbchem.a131988. [DOI] [PubMed] [Google Scholar]
- Trybus K. M., Huiatt T. W., Lowey S. A bent monomeric conformation of myosin from smooth muscle. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6151–6155. doi: 10.1073/pnas.79.20.6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachsberger P. R., Pepe F. A. Interaction between vertebrate skeletal and uterine muscle myosins and light meromyosins. J Cell Biol. 1980 Apr;85(1):33–41. doi: 10.1083/jcb.85.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeds A. G., Pope B. Studies on the chymotryptic digestion of myosin. Effects of divalent cations on proteolytic susceptibility. J Mol Biol. 1977 Apr;111(2):129–157. doi: 10.1016/s0022-2836(77)80119-8. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Taylor R. S. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975 Sep 4;257(5521):54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]
- Yagi N., Offer G. W. X-ray diffraction and electron microscopy of a light meromyosin tactoid. J Mol Biol. 1981 Sep 25;151(3):467–490. doi: 10.1016/0022-2836(81)90006-1. [DOI] [PubMed] [Google Scholar]