Abstract
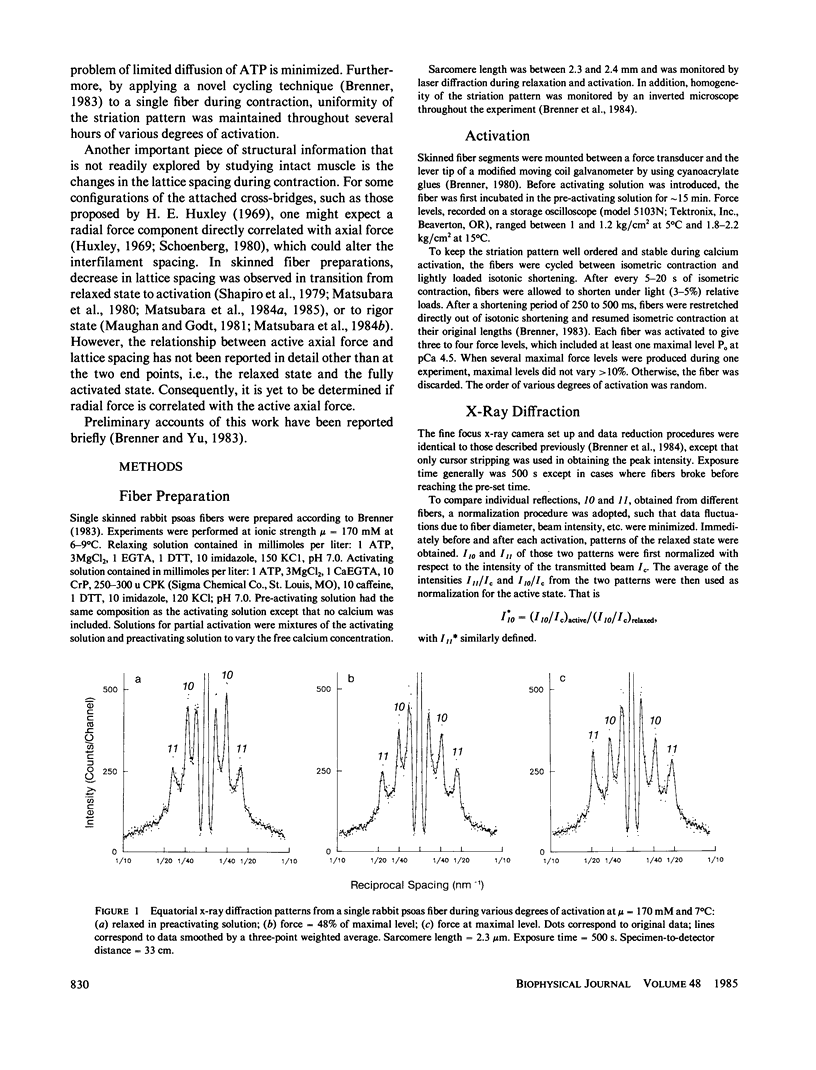
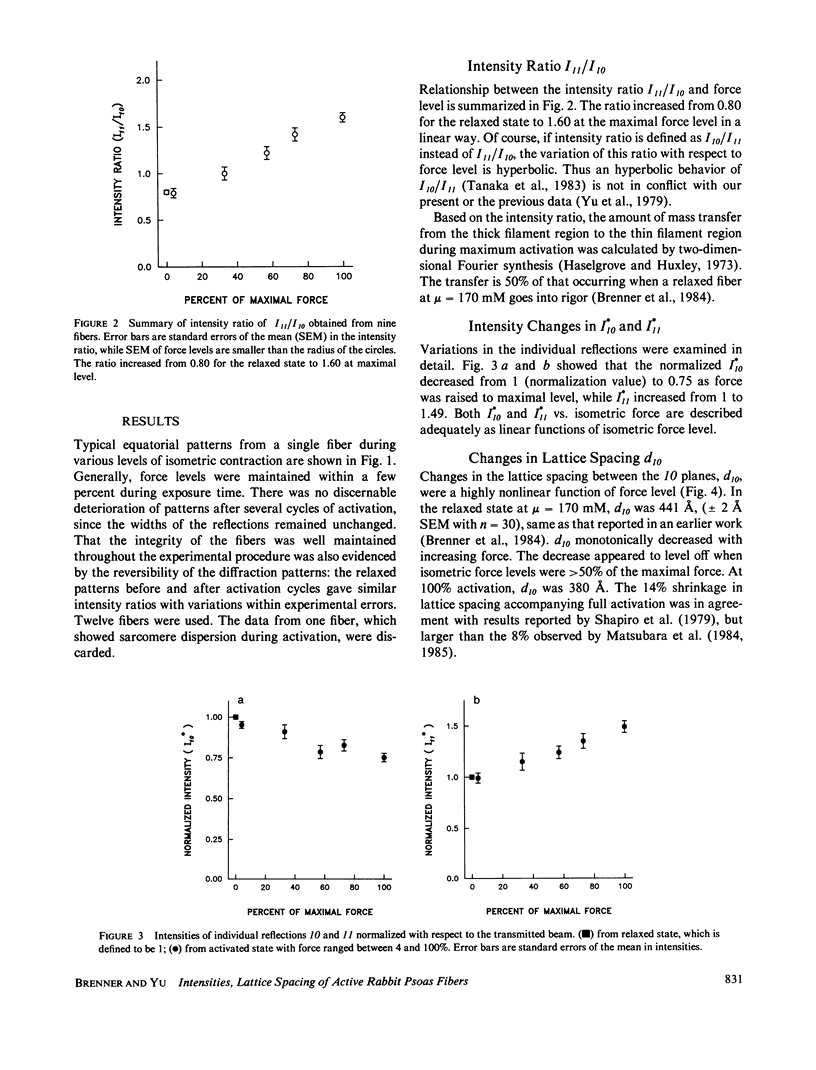
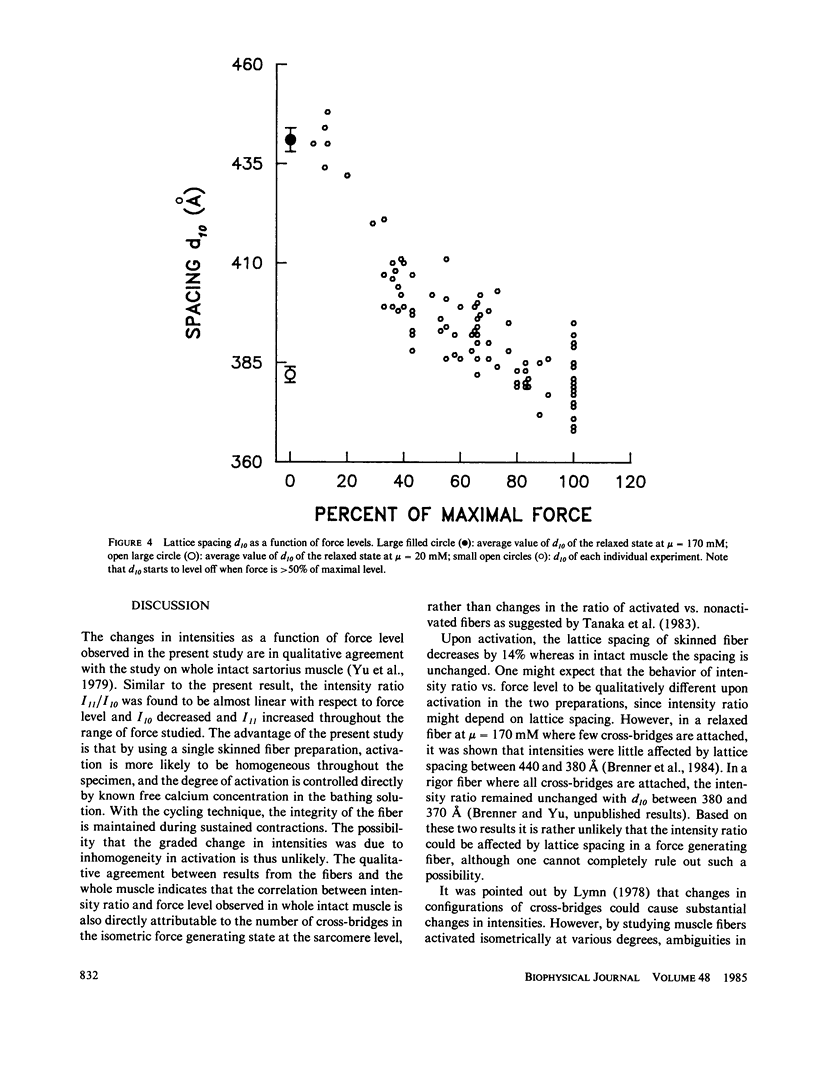
Equatorial x-ray diffraction patterns were obtained from single skinned rabbit psoas fibers during various degrees of activation under isometric conditions at ionic strength 170 mM and 6-9 degrees C. By direct calcium activation, contraction was homogeneous throughout the preparation, and by using a cycling technique (Brenner, 1983) integrity of the fiber was maintained even during prolonged steady activation. The intensity ratio of the two innermost reflections I11/I10, and the normalized intensities I*10 and I*11 varied linearly with increasing force. Thus the result agreed qualitatively with an earlier finding, obtained from the whole sartorius muscle, that intensity changes in 10 and 11 are directly correlated with isometric force level (Yu et al., 1979). Spacing of the myofilament lattice (d10) was found to decrease with increasing isometric tension. With the filaments in full overlap, maximum shrinkage was 14%. The lattice spacing started to level off when the degree of calcium activation was greater than or equal to 50%, approaching a limit approximately at 380-360 A. This decrease of the lattice spacing indicates that there is a radial force produced by force generating cross-bridges, but the net radial force appears to become insignificant as lattice spacing approaches 380-360 A.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenner B., Schoenberg M., Chalovich J. M., Greene L. E., Eisenberg E. Evidence for cross-bridge attachment in relaxed muscle at low ionic strength. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7288–7291. doi: 10.1073/pnas.79.23.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. Technique for stabilizing the striation pattern in maximally calcium-activated skinned rabbit psoas fibers. Biophys J. 1983 Jan;41(1):99–102. doi: 10.1016/S0006-3495(83)84411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B., Yu L. C., Podolsky R. J. X-ray diffraction evidence for cross-bridge formation in relaxed muscle fibers at various ionic strengths. Biophys J. 1984 Sep;46(3):299–306. doi: 10.1016/S0006-3495(84)84026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman Y. E., Simmons R. M. Control of sarcomere length in skinned muscle fibres of Rana temporaria during mechanical transients. J Physiol. 1984 May;350:497–518. doi: 10.1113/jphysiol.1984.sp015215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselgrove J. C., Huxley H. E. X-ray evidence for radial cross-bridge movement and for the sliding filament model in actively contracting skeletal muscle. J Mol Biol. 1973 Jul 15;77(4):549–568. doi: 10.1016/0022-2836(73)90222-2. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. Structural difference between resting and rigor muscle; evidence from intensity changes in the lowangle equatorial x-ray diagram. J Mol Biol. 1968 Nov 14;37(3):507–520. doi: 10.1016/0022-2836(68)90118-6. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Lymn R. W. Myosin subfragment-1 attachment to actin. Expected effect on equatorial reflections. Biophys J. 1978 Jan;21(1):93–98. doi: 10.1016/S0006-3495(78)85510-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara I., Goldman Y. E., Simmons R. M. Changes in the lateral filament spacing of skinned muscle fibres when cross-bridges attach. J Mol Biol. 1984 Feb 15;173(1):15–33. doi: 10.1016/0022-2836(84)90401-7. [DOI] [PubMed] [Google Scholar]
- Matsubara I., Umazume Y., Yagi N. Lateral filamentary spacing in chemically skinned murine muscles during contraction. J Physiol. 1985 Mar;360:135–148. doi: 10.1113/jphysiol.1985.sp015608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan D. W., Godt R. E. Radial forces within muscle fibers in rigor. J Gen Physiol. 1981 Jan;77(1):49–64. doi: 10.1085/jgp.77.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky R. J., St Onge H., Yu L., Lymn R. W. X-ray diffraction of actively shortening muscle. Proc Natl Acad Sci U S A. 1976 Mar;73(3):813–817. doi: 10.1073/pnas.73.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky R. J., Teichholz L. E. The relation between calcium and contraction kinetics in skinned muscle fibres. J Physiol. 1970 Nov;211(1):19–35. doi: 10.1113/jphysiol.1970.sp009263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberg M. Geometrical factors influencing muscle force development. II. Radial forces. Biophys J. 1980 Apr;30(1):69–77. doi: 10.1016/S0006-3495(80)85077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire J. M. Structure and force generation in muscle. Nature. 1979 Sep 13;281(5727):99–100. doi: 10.1038/281099a0. [DOI] [PubMed] [Google Scholar]
- Yu L. P., Hartt J. E., Podolsky R. J. Equatorial x-ray intensities and isometric force levels in frog sartorius muscle. J Mol Biol. 1979 Jul 25;132(1):53–67. doi: 10.1016/0022-2836(79)90495-9. [DOI] [PubMed] [Google Scholar]


