Abstract
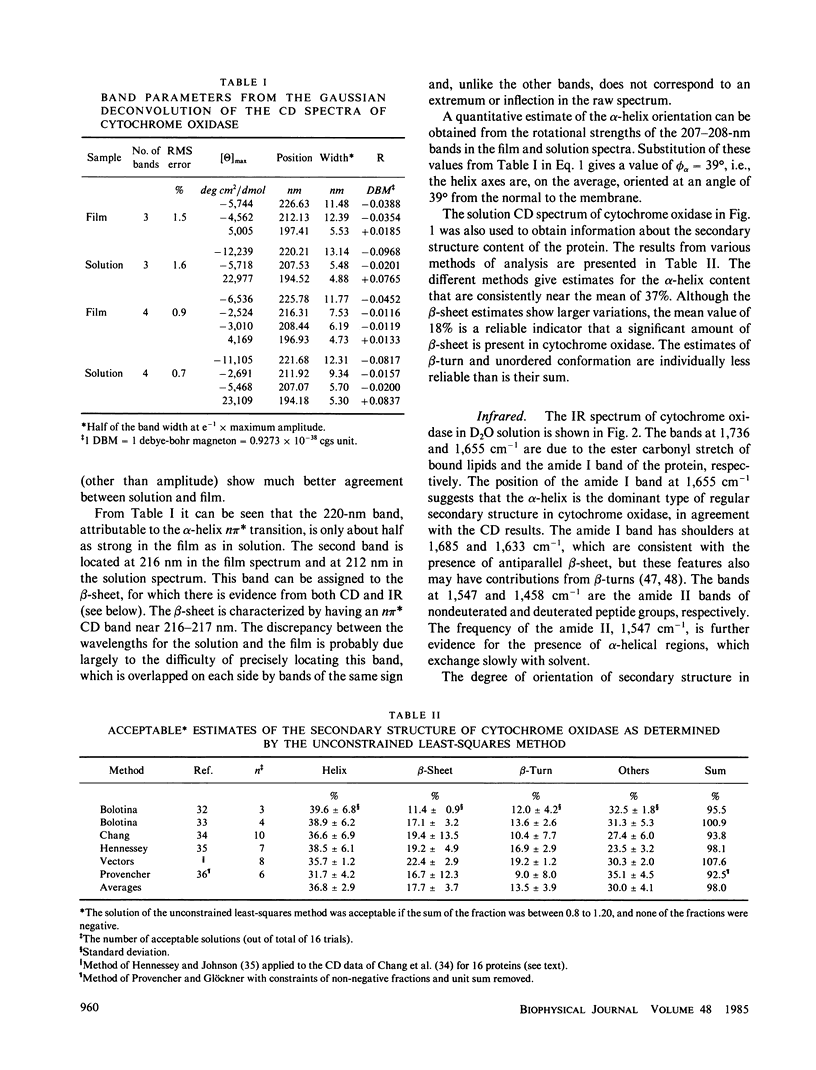
The circular dichroism (CD) of cytochrome oxidase in solution indicates the presence of both alpha-helix (approximately 37%) and B-sheet (approximately 18%). In oriented films generated by the isopotential spin-dry method, the CD measured normal to the film shows a marked decrease in the negative bands at 222 and 208 nm, and a decrease and red shift in the positive band near 195 nm, relative to solution spectra. These features are characteristic of alpha-helices oriented with their helix axes along the direction of light propagation. A quantitative estimate of the orientation, based on the ratio of the rotational strengths of the 208-nm band in the film and in solution, leads to an average angle between the helix axis and the normal to the film, phi alpha of approximately 39 degrees. A method for analyzing infrared (IR) linear dichroism is developed that can be applied to proteins with comparable amounts of alpha-helix and beta-sheet. From analysis of the amide I band, phi alpha is found to lie between 20 and 36 degrees, depending on the angle that the amide I transition moment forms with the helix axis. A survey of the literature on the amide I transition moment direction indicates that a value of approximately 27 degrees is appropriate for standard alpha-helical systems, such as those in cytochrome oxidase. A larger value, near 40 degrees, is reasonable for systems that have distorted alpha-helices, as evidenced by amide I frequencies above 1,660 cm-1, as is the case of bacteriorhodopsin. This conclusion supports phi alpha approximately 36 degrees from IR linear dichroism, in agreement with the CD results. Linear dichroism in the amide I and amide II region indicates that the beta-sheet in cytochrome oxidase is oriented with the carbonyl groups nearly parallel to the plane of the membrane and the chain direction inclined at approximately 40 degrees to the normal. Comparison of these results with tentative identification of transmembrane helices from sequence data suggests that either some of the transmembrane helices are inclined at an unexpectedly large angle to the normal, or the number of such helices has been overestimated. Some putative transmembrane helices may be beta-strands spanning the membrane.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Azzi A. Cytochrome c oxidase. Towards a clarification of its structure, interactions and mechanism. Biochim Biophys Acta. 1980 Dec;594(4):231–252. doi: 10.1016/0304-4173(80)90002-6. [DOI] [PubMed] [Google Scholar]
- BRADBURY E. M., BROWN L., DOWNIE A. R., ELLIOTT A., FRASER R. D., HANBY W. E. The structure of the omegaform of poly-Beta-benzyl-L-aspartate. J Mol Biol. 1962 Aug;5:230–247. doi: 10.1016/s0022-2836(62)80086-2. [DOI] [PubMed] [Google Scholar]
- Bandekar J., Krimm S. Vibrational analysis of peptides, polypeptides, and proteins: Characteristic amide bands of beta-turns. Proc Natl Acad Sci U S A. 1979 Feb;76(2):774–777. doi: 10.1073/pnas.76.2.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasie J. K., Erecińska M., Samuels S., Leigh J. S. The structure of a cytochrome oxidase-lipid model membrane. Biochim Biophys Acta. 1978 Jan 11;501(1):33–52. doi: 10.1016/0005-2728(78)90093-2. [DOI] [PubMed] [Google Scholar]
- Capaldi R. A. A hydrogen-deuterium exchange study of membranous cytochrome oxidase. Biochim Biophys Acta. 1973 Apr 20;303(2):237–241. doi: 10.1016/0005-2795(73)90352-8. [DOI] [PubMed] [Google Scholar]
- Capaldi R. A. Arrangement of proteins in the mitochondrial inner membrane. Biochim Biophys Acta. 1982 Nov 30;694(3):291–306. doi: 10.1016/0304-4157(82)90009-0. [DOI] [PubMed] [Google Scholar]
- Capaldi R. A., Malatesta F., Darley-Usmar V. M. Structure of cytochrome c oxidase. Biochim Biophys Acta. 1983 Jul 15;726(2):135–148. doi: 10.1016/0304-4173(83)90003-4. [DOI] [PubMed] [Google Scholar]
- Chang C. T., Wu C. S., Yang J. T. Circular dichroic analysis of protein conformation: inclusion of the beta-turns. Anal Biochem. 1978 Nov;91(1):13–31. doi: 10.1016/0003-2697(78)90812-6. [DOI] [PubMed] [Google Scholar]
- Clark N. A., Rothschild K. J., Luippold D. A., Simon B. A. Surface-induced lamellar orientation of multilayer membrane arrays. Theoretical analysis and a new method with application to purple membrane fragments. Biophys J. 1980 Jul;31(1):65–96. doi: 10.1016/S0006-3495(80)85041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deatherage J. F., Henderson R., Capaldi R. A. Relationship between membrane and cytoplasmic domains in cytochrome c oxidase by electron microscopy in media of different density. J Mol Biol. 1982 Jul 5;158(3):501–514. doi: 10.1016/0022-2836(82)90211-x. [DOI] [PubMed] [Google Scholar]
- Deatherage J. F., Henderson R., Capaldi R. A. Three-dimensional structures of cytochrome c oxidase vesicle crystals in negative stain. J Mol Biol. 1982 Jul 5;158(3):487–499. doi: 10.1016/0022-2836(82)90210-8. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Hennessey J. P., Jr, Johnson W. C., Jr Information content in the circular dichroism of proteins. Biochemistry. 1981 Mar 3;20(5):1085–1094. doi: 10.1021/bi00508a007. [DOI] [PubMed] [Google Scholar]
- Heyn M. P., Cherry R. J., Müller U. Transient and linear dichroism studies on bacteriorhodopsin: determination of the orientation of the 568 nm all-trans retinal chromophore. J Mol Biol. 1977 Dec 15;117(3):607–620. doi: 10.1016/0022-2836(77)90060-2. [DOI] [PubMed] [Google Scholar]
- Jap B. K., Maestre M. F., Hayward S. B., Glaeser R. M. Peptide-chain secondary structure of bacteriorhodopsin. Biophys J. 1983 Jul;43(1):81–89. doi: 10.1016/S0006-3495(83)84326-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadenbach B., Merle P. On the function of multiple subunits of cytochrome c oxidase from higher eukaryotes. FEBS Lett. 1981 Nov 30;135(1):1–11. doi: 10.1016/0014-5793(81)80932-5. [DOI] [PubMed] [Google Scholar]
- Krimm S., Dwivedi A. M. Infrared spectrum of the purple membrane: clue to a proton conduction mechanism? Science. 1982 Apr 23;216(4544):407–408. doi: 10.1126/science.6280277. [DOI] [PubMed] [Google Scholar]
- Leifer D., Henderson R. Three-dimensional structure of orthorhombic purple membrane at 6.5 A resolution. J Mol Biol. 1983 Jan 25;163(3):451–466. doi: 10.1016/0022-2836(83)90068-2. [DOI] [PubMed] [Google Scholar]
- Michel-Villaz M., Saibil H. R., Chabre M. Orientation of rhodopsin alpha-helices in in retinal rod outer segment membranes studied by infrared linear dichroism. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4405–4408. doi: 10.1073/pnas.76.9.4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muccio D. D., Cassim J. Y. Interpretation of the absorption and circular dichroic spectra of oriented purple membrane films. Biophys J. 1979 Jun;26(3):427–440. doi: 10.1016/S0006-3495(79)85263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer Y. P. A new method for the conformational analysis of proteins and polypeptides from circular dichroism spectra. Res Commun Chem Pathol Pharmacol. 1970 Sep;1(5):607–616. [PubMed] [Google Scholar]
- Myer Y. P. Conformation of cytochromes. V. Cytochrome c oxidase. J Biol Chem. 1971 Mar 10;246(5):1241–1248. [PubMed] [Google Scholar]
- Nabedryk E., Breton J. Orientation of intrinsic proteins in photosynthetic membranes. Polarized infrared spectroscopy of chloroplasts and chromatophores. Biochim Biophys Acta. 1981 May 13;635(3):515–524. doi: 10.1016/0005-2728(81)90110-9. [DOI] [PubMed] [Google Scholar]
- Némethy G., Phillips D. C., Leach S. J., Scheraga H. A. A second right-handed helical structure with the parameters of the Pauling-Corey alpha-helix. Nature. 1967 Apr 22;214(5086):363–365. doi: 10.1038/214363a0. [DOI] [PubMed] [Google Scholar]
- Provencher S. W., Glöckner J. Estimation of globular protein secondary structure from circular dichroism. Biochemistry. 1981 Jan 6;20(1):33–37. doi: 10.1021/bi00504a006. [DOI] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Rothschild K. J., Clark N. A. Anomalous amide I infrared absorption of purple membrane. Science. 1979 Apr 20;204(4390):311–312. doi: 10.1126/science.432645. [DOI] [PubMed] [Google Scholar]
- Rothschild K. J., Clark N. A. Polarized infrared spectroscopy of oriented purple membrane. Biophys J. 1979 Mar;25(3):473–487. doi: 10.1016/S0006-3495(79)85317-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild K. J., Clark N. A., Rosen K. M., Sanches R., Hsiao T. L. Spectroscopic study of photoreceptor membrane incorporated into a multilamellar film. Biochem Biophys Res Commun. 1980 Feb 27;92(4):1266–1272. doi: 10.1016/0006-291x(80)90423-4. [DOI] [PubMed] [Google Scholar]
- Rothschild K. J., Rosen K. M., Clark N. A. Incorporation of photoreceptor membrane into a multilamellar film. Biophys J. 1980 Jul;31(1):45–52. doi: 10.1016/S0006-3495(80)85039-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild K. J., Sanches R., Hsiao T. L., Clark N. A. A spectroscopic study of rhodopsin alpha-helix orientation. Biophys J. 1980 Jul;31(1):53–64. doi: 10.1016/S0006-3495(80)85040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior A. E. Secondary and tertiary structure of membrane proteins involved in proton translocation. Biochim Biophys Acta. 1983 Jul 15;726(2):81–95. doi: 10.1016/0304-4173(83)90001-0. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Woody R. W. Optical and other properties of a hydrocarbon-soluble polypeptide, poly-gamma-(N-dodecyl)-L-glutamate. Biopolymers. 1973 Dec;12(12):2657–2665. doi: 10.1002/bip.1973.360121203. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Haniu M., Yasunobu K. T. Amino acid sequence of the heme a subunit of bovine heart cytochrome oxidase and sequence homology with hemoglobin. Biochem Biophys Res Commun. 1977 Jun 20;76(4):1014–1019. doi: 10.1016/0006-291x(77)90957-3. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Bustamante C., Maestre M. F. The optical activity of nucleic acids and their aggregates. Annu Rev Biophys Bioeng. 1980;9:107–141. doi: 10.1146/annurev.bb.09.060180.000543. [DOI] [PubMed] [Google Scholar]
- Urry D. W. Protein conformation in biomembranes: optical rotation and absorption of membrane suspensions. Biochim Biophys Acta. 1972 Feb 14;265(1):115–168. doi: 10.1016/0304-4157(72)90021-4. [DOI] [PubMed] [Google Scholar]
- VAN GELDERB, SLATER E. C. TITRATION OF CYTOCHROME C OXIDASE WITH NADH AND PHENAZINE METHOSULPHATE. Biochim Biophys Acta. 1963 Aug 6;73:663–665. doi: 10.1016/0006-3002(63)90342-1. [DOI] [PubMed] [Google Scholar]
- Yoshikawa S., Choc M. G., O'Toole M. C., Caughey W. S. An infrared study of CO binding to heart cytochrome c oxidase and hemoglobin A. Implications re O2 reactions. J Biol Chem. 1977 Aug 10;252(15):5498–5508. [PubMed] [Google Scholar]


