Abstract
Chagas' disease results from infection with Trypanosoma cruzi, a protozoan parasite that establishes systemic intracellular infection after mucosal invasion. We hypothesized that ideal vaccines for mucosally invasive, intracellular pathogens like T. cruzi should induce mucosal type 2 immunity for optimal induction of protective secretory immunoglobulin A (IgA) and systemic type 1 immunity protective against intracellular replication. However, differential mucosal and systemic immune memory could be difficult to induce because of reciprocal inhibitory actions between type 1 and type 2 responses. To test our hypotheses, we investigated the protective effects of type 1 and type 2 biased vaccines against mucosal and systemic T. cruzi challenges. Intranasal vaccinations were given with recombinant interleukin-12 (IL-12)- and IL-4-neutralizing antibody (Ab) for type 1 immune bias, or recombinant IL-4 and gamma interferon-neutralizing Ab for type 2 immune bias. Cytokine RNA and protein studies confirmed that highly polarized memory immune responses were induced by our vaccination protocols. Survival after virulent subcutaneous T. cruzi challenge was used to assess systemic protection. Mucosal protection was assessed by measuring the relative inhibition of parasite replication in mucosal tissues early after oral T. cruzi challenge, using both PCR and quantitative culture techniques. As expected, only type 1 responses protected against systemic challenges (P < 0.01). However, contrary to our original hypothesis, type 1 responses optimally protected against mucosal challenges as well (P < 0.05). Type 1 and type 2 biased vaccines induced similar secretory IgA responses. We conclude that future vaccines for T. cruzi and possibly other mucosally invasive, intracellular pathogens should induce both mucosal and systemic type 1 immunity.
Many human infectious diseases for which effective vaccines have not been developed initiate infection by mucosal invasion but then establish chronic states of intracellular parasitism (e.g., tuberculosis, AIDS, and many other chronic viral and parasitic diseases). The induction of both mucosal and systemic immune resistance could maximize protection against diseases caused by mucosally invasive, intracellular pathogens. However, it is unknown whether optimal mucosal and systemic immunity protective against these pathogens can be induced concurrently. Mucosal and systemic protection may require different immune responses. T cells producing interleukin-4 (IL-4), IL-5, and IL-10 (type 2 phenotype) or producing high levels of transforming growth factor-β (type 3 phenotype) may be important for induction of secretory immunoglobulin A (IgA) responses protective against mucosal infection (15, 32). On the other hand, T cells producing gamma interferon (IFN-γ), tumor necrosis factor alpha, and IL-2 (type 1 phenotype) are clearly protective against systemic intracellular replication of many human pathogens (1, 26). Type 1 and type 2/3 responses have reciprocal inhibitory activities that may present major obstacles for the development of vaccines designed to induce differential T-cell responses in mucosal and systemic immune compartments (7, 9,12-14). Therefore, it is of critical importance for the field of vaccine immunobiology to define the specific mucosal and systemic immune responses protective against mucosally invasive, intracellular pathogens and to study the interactions between protective mucosal and systemic responses.
Trypanosoma cruzi is an appropriate model for studies of the molecular and cellular interactions between mucosal and systemic immune responses directed against mucosally invasive, intracellular pathogens. Chagas' disease in humans can occur during acute or chronic T. cruzi infection, after either mucosal or systemic routes of transmission (5, 11, 16, 20, 28). Infective insect-derived metacyclic trypomastigotes (IMT) are excreted onto the surface of mammalian hosts by infected reduviid bugs, initiating infection by contamination of open wounds or mucosal tissues. Transmission by conjunctival, oral, and subcutaneous routes of IMT infection can occur. T. cruzi blood-form trypomastigotes (BFT) can be transmitted via contaminated blood products as well. Therefore, both mucosal and systemic immunity could be important for vaccines designed to prevent Chagas' disease.
We have developed the unique methodology required to study T. cruzi replication and parasite-specific immunity in mucosal and systemic tissues independently (17). We identified gastric epithelial invasion as the anatomical route of infection after oral IMT challenge (50% infectious dose = 20 IMT), can study T. cruzi-specific mucosal immunity in lymphocytes isolated from gastric epithelium and draining lymph nodes, and can quantify T. cruzi replication in gastric tissues after oral IMT challenges to determine the relative levels of mucosal protection afforded by different vaccine approaches. Systemic immune protection can be studied independently by using standard virulent BFT challenge protocols and following survival postchallenge.
In addition, identification of key cytokines required for generation of different T-cell subsets has made it possible to induce highly polarized antigen-specific type 1 and type 2 responses in vitro and in vivo. IL-12 and IL-4 are critical for induction of type 1 and type 2 responses, respectively (26). Kumar et al. reported that in vitro-generated CD4+ Th1 but not Th2 cells could adoptively transfer protection against systemic T. cruzi challenges (23). Our group demonstrated that recombinant IL-12 and anti-IL-4 induced type 1 biased responses in vivo that were highly protective against normally lethal T. cruzi systemic challenges (19). IL-4 plus anti-IFN-γ induced type 2 polarized responses that failed to protect against systemic T. cruzi challenges (19). The protective effects of highly polarized type 1 and type 2 responses against mucosal T. cruzi challenges have not been studied previously.
In the present work, we directly examined the protective effects of T. cruzi-specific type 1 and type 2 responses against both mucosal and systemic protection. These studies are relevant for efforts to develop vaccines against several important mucosally invasive, chronic intracellular pathogens.
MATERIALS AND METHODS
Parasites and mice.
The Tulahuén strain of T. cruzi and BALB/c mice (Harlan Sprague-Dawley Inc., Indianapolis, Ind.) were used in these experiments. For systemic challenges, heparinized blood was obtained from heavily parasitemic mice infected 2 weeks previously with BFT. Experimental mice were challenged with 5,000 BFT subcutaneously in the base of the tail. Mortality was followed for greater than 3 months postchallenge. IMT were prepared by allowing T. cruzi-infected reduviid bugs (Dipetalogaster maximus) to feed on anesthetized mice. Engorged insects were then incubated in glass vials for 3 to 5 h, excreta were pooled, and the IMT concentration was determined by direct hemocytometer count. For oral IMT challenges, mice were kept without food and water for at least 4 h prechallenge and 15 min postchallenge. Mice were treated intragastrically with 0.5 ml of 1.5% sodium bicarbonate in Hanks buffer 15 min prior to oral administration of 10,000 IMT. Levels of mucosal tissue parasitism were studied during the first 2 weeks after IMT oral challenge.
Immunizations.
Table 1 schematically presents the different immunization protocols used to induce T. cruzi-specific nonbiased, type 1 biased, and type 2 biased responses. These immunization protocols are similar to those reported in our group's previous manuscript demonstrating that type 1 subcutaneous vaccinations provide systemic T. cruzi protection, except that we now have adapted these protocols for intranasal vaccination. BALB/c mice (6 to 8 weeks old) were immunized intranasally with 20 μg of T. cruzi lysate and 2.5 μg of cholera toxin (Sigma, St. Louis, Mo.). Mice given antigen and cholera toxin alone were defined as the Th0 group. To bias for type 1 responses, mice received 1-μg doses of recombinant murine IL-12 (provided by Stan Wolf, Genetics Institute) intraperitoneally for three successive days beginning the day before intranasal immunization. In addition, type 1 biased mice were injected intraperitoneally with 0.5 mg of the anti-IL-4 monoclonal antibody 11B11 the day before and 2 days after intranasal immunization. Mice given the type 2 bias immunization protocol received 2,500 U of recombinant murine IL-4 (Pharmingen, San Diego, Calif.) and 0.5 mg of the anti-IFN-γ monoclonal antibody R46A2 instead of IL-12 and 11B11. All three groups of mice (nonbiased, type 1 biased, and type 2 biased) were revaccinated 2 weeks later with the same treatments given during their first vaccination. Two weeks after the second vaccination all mice received 20 μg of T. cruzi lysate alone intranasally. Immunized mice were harvested 3 days after the last antigen administration for evaluation of immune responses and challenged with T. cruzi 2 to 6 weeks after their last booster vaccination.
TABLE 1.
Type 1, type 2, and nonbiased vaccination protocolsa
| Day | Nonbiased | Type 1 bias | Type 2 bias |
|---|---|---|---|
| −1 | IL-12 and anti-IL-4 IP | IL-4 and anti-IFN-γ IP | |
| 0 | Tc lysate + CT IN | Tc lysate + CT IN, IL-12 IP | Tc lysate + CT IN, IL-4 IP |
| 1 | IL-12 IP | IL-4 IP | |
| 2 | Anti-IL-4 IP | Anti-IFN-γ IP | |
| 13 | IL-12 and anti-IL-4 IP | IL-4 and anti-IFN-γ IP | |
| 14 | Tc lysate + CT IN | Tc lysate + CT IN, IL-12 IP | Tc lysate + CT IN, IL-4 IP |
| 15 | IL-12 IP | IL-4 IP | |
| 16 | Anti-IL-4 IP | Anti-IFN-γ IP | |
| 28 | Tc lysate IN | Tc lysate IN | Tc lysate IN |
| 56 | BFT or IMT challenge | BFT or IMT challenge | BFT or IMT challenge |
Tc lysate, whole T. cruzi lysate; CT, cholera toxin; IN, intranasally; IP, intraperitoneally; BFT, blood form trypomastigote; IMT, insect-derived metacyclic trypomastigote. BALB/c mice were immunized with 20 μg of Tc lysate and 2.5 μg of CT IN. Type 1 biased mice received 1 μg of recombinant IL-12 IP for 3 consecutive days, and 0.5 mg of the IL-4-neutralizing antibody 11B11 IP twice for each vaccination as indicated. Mice given the type 2 bias received 2,500 U of IL-4 and 0.5 mg of the IFN-γ-neutralizing antibody R46A2 IP instead of IL-12 and 11B11, respectively. Identical booster vaccination schedules were given to each of the three groups 2 weeks later, and then final IN vaccinations with Tc lysate alone were given to all groups 2 weeks after the second immunization. Immune responses were studied 3 days after the final immunizations. Mice were challenged subcutaneously or orally with 5,000 BFT or 10,000 IMT, respectively, 4 weeks following their final IN immunization.
Antigen-specific lymphoproliferation.
Total spleen cell suspensions (2 × 105 cells/200 μl) were stimulated with 2 and 20 μg of T. cruzi lysate/ml in round-bottom 96-well plates. CD4+ T cells were purified as recommended by the manufacturer by using immunomagnetic positive selection with Miltenyi anti-CD4 microbeads (Miltenyi Biotec, Auburn, Calif.). Purified CD4+ spleen cells (5 × 104/200 μl) were cultured in 96-well plates with irradiated syngeneic spleen cells (106/200 μl) and pulsed with T. cruzi lysate. After 72 h of in vitro stimulation, these cultures were pulsed for 4 to 6 h with 0.5 μCi of [3H]thymidine (Amersham, Piscataway, N.J.) and then harvested using a Skatron semiautomated cell harvester. Filter disks were scintillated with Wallac OptiScint HiSafe and counted with a Taurus automatic liquid scintillation counter (ICN Biomedical, Huntsville, Ala.).
Measurements of T-cell cytokine mRNA expression.
Total spleen cells (4 × 106) from immunized mice were stimulated in 24-well plates for 24 to 48 h with medium alone, 20 μg of T. cruzi lysate/ml, or 1 μg of concanavalin A (ConA) (Sigma) per ml. Total RNA samples were harvested from these cultures using a commercially available RNeasy kit (Qiagen, Valencia, Calif.). RiboQuant multiprobe RNase protection assays were performed according to the manufacturer's guidelines (Pharmingen) to assess cytokine mRNA expression levels. Briefly, synthesized 32P-labeled RNA probes specific for the murine cytokine genes of interest (mCK-1 multiprobe template set) were hybridized to 2.5-μg samples of RNA. After RNase treatment and cleanup of RNA, samples were loaded and run on Novex QuickPoint mini-sequencing gels (Pharmingen) and visualized using a PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.). L32 and glyceraldehyde-3-phosphate dehydrogenase RNA probes were included as controls for estimating constitutive levels of mRNA expression (L32 bands on radiograms were used to normalize densitometry data).
Measurements of T-cell cytokine secretion.
IFN-γ and IL-4 were measured by antibody-capture enzyme-linked immunosorbent assay (ELISA) as previously described (18) in cell culture supernatants collected 72 h after in vitro stimulation. Background cytokine levels present in cultures rested in medium alone were subtracted from matching antigen-stimulated values. These background levels were always less than 10 U/ml.
Mucosal IgA ELISA.
Fecal pellets were collected from mice at various times pre- and postimmunization. Fecal extracts were prepared from individual mice or from pools of multiple mice in independent groups. Fecal extracts were suspended in phosphate-buffered saline (PBS) plus 10% fetal calf serum (FCS) (200 mg of fecal pellets/ml of PBS), vortexed at 4°C for 1 h, clarified by centrifugation, and studied in T. cruzi-specific IgA ELISAs. Immulon-2 plates were coated with 10 μg of T. cruzi lysate/ml and blocked with PBS plus 10% FCS, and then fecal samples were added in serial twofold dilutions. ELISA plates were developed by sequential addition of biotinylated anti-mouse IgA (Southern Biotechnology Associates, Inc., Birmingham, Ala.), streptavidin-horseradish peroxidase (Jackson Immunologicals, West Grove, Pa)., and 3,3′,5,5′-tetramethylbenzidine substrate. Enzymatic reactions were stopped with H2SO4 and ELISA optical densities (ODs) were read at 450 nm with a reference OD of 540 nm.
SQ-PCR.
Mice were sacrificed 10 days after oral IMT challenge, stomachs were removed by cutting esophageal and pyloric attachments, and these organs were opened along the greater curvature and flushed with sterile PBS. Gastric tissue DNA samples were purified using a commercially available QIAamp kit (Qiagen), and total DNA concentrations were adjusted to 50 ng/μl (as determined by the OD260). Constant concentrations of gastric DNA samples were added to PCRs spiked with titrations of a positive control DNA template using primers amplifying a 418-bp C-terminal fragment of the gene for cruzipain, the T. cruzi major cysteinyl protease (5′ primer, GCGGGATCCCATGGGTCCCGGACCCACTCCC; 3′ primer, GCGGGATCCCCCTCAGAGACGGCGATGACGGCT). The positive control DNA template was engineered by PCR addition of the C-terminal cruzipain primer sequences to the ends of a 329-bp fragment of the bacterial ampicillin resistance gene encoded by pNEB (New England Biolabs, Inc., Beverly, Mass.). The amplified positive control product is 25 bp shorter than the wild-type T. cruzi PCR product. The positive control was quantified in terms of the relative T. cruzi molecular equivalents (ME) per microliter in semiquantitative PCR (SQ-PCR) experiments using DNA harvested from a known concentration of T. cruzi epimastigotes grown in pure culture. The optimized buffer used for SQ-PCR included 60 mM Tris (pH 9.0), 3.5 mM MgCl2, 15 mM (NH4)2SO4, a 200 μM concentration of deoxynucleoside triphosphates and 10 pmol of each primer. After 2.5 min of an initial 98°C hot start and 35 PCR amplification cycles (95°C for 1 min followed by 65°C for 2.5 min), reaction products were separated in 2% Tris-borate-EDTA (TBE) agarose gels and ethidium bromide stained. Reactions showing equivalent intensity of positive control and native C-terminal cruzipain bands were used to estimate T. cruzi ME present in unknown DNA samples.
Quantitation of viable T. cruzi mucosal parasitism.
Mucosal tissue parasitism in different vaccination groups was investigated after oral IMT challenge as described previously (17). Briefly, titrations of gastric lymph node cells plated in 96-well microtiter plates with 200 μl of liver infusion tryptose broth (Oxoid, Ltd., Basingstoke, Hampshire, England) containing 0.02 mg of hemin/ml, 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 10% FCS (LDNT+ [6, 21]), were incubated at 26°C for 2 months. Every 2 weeks wells were inspected by inverted light microscopy for evidence of T. cruzi epimastigotes. The minimal mononuclear cell concentrations associated with epimastigote outgrowth were recorded and used to estimate initial parasite concentrations in mucosal tissues with the following equation: parasites per million mononuclear cells = (1 million)/(minimal mononuclear cell concentration with parasites).
RESULTS
Similar T. cruzi-specific lymphoproliferative responses were induced by all intranasal immunization protocols.
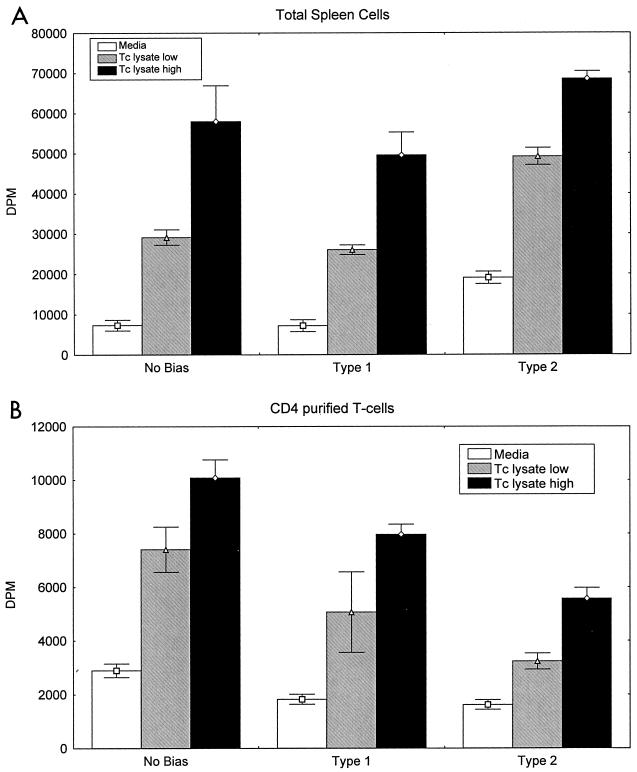
We studied antigen-specific lymphoproliferative responses in total spleen cells harvested from intranasally immunized mice and found that T. cruzi lysate induced similar levels of antigen-specific proliferation regardless of whether type 1, type 2, or no immune bias was used (Fig. 1A). We also studied antigen-specific lymphoproliferation in highly purified splenic CD4+ T cells from all groups of T. cruzi-immunized mice (Fig. 1B). CD4+ T cells purified by positive immunomagnetic selection as described in Materials and Methods were greater than 95% CD3/CD4 double-positive by fluorescence-activated cell sorter analysis (results not shown). These purified cells were cultured with irradiated spleen cells from naïve mice as antigen-presenting cells and stimulated with T. cruzi lysate (Fig. 1B). Similar to total spleen cells, CD4+ T cells from all groups were shown to develop similar T. cruzi-specific lymphoproliferative responses. Lymphoproliferative responses were not detected in naïve total or purified CD4+ splenic T cells after stimulation with T. cruzi lysate or in spleen cells or purified CD4+ T cells from immunized mice rested in medium alone (data not shown). These results indicate that similar levels of overall lymphocyte activation were induced by all three intranasal immunization protocols.
FIG. 1.
Intranasal vaccinations with no bias, type 1 bias, and type 2 bias induce similar T. cruzi-specific lymphoproliferation. Mice were immunized as described in Table 1, and spleen cells were harvested 3 days following the final intranasal immunization. (A) Total spleen cells (2 × 105/200 μl) were stimulated with 2 or 20 μg of T. cruzi lysate/ml for 72 h prior to measurement of [3H]thymidine incorporation. (B) A total of 5 × 104 CD4+ purified spleen cells/well and 106 irradiated syngeneic total spleen cells/well were stimulated in 96-well plates with 2 or 20 μg of T. cruzi lysate/ml for 72 h prior to measurement of [3H]thymidine incorporation. Shown are the means and standard errors from a single experiment with pooled cells from two to three mice/group. These results are representative of at least five similar experiments.
Nonbiased, type 1 biased, and type 2 biased immunization protocols induce differential cytokine response profiles.
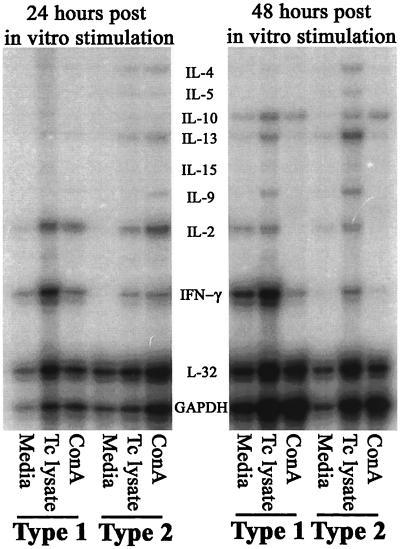
The induction of differential cytokine response profiles in mice immunized with type 1 and type 2 bias was evaluated in detail at the mRNA level. RNA samples collected from total spleen cells 24 and 48 h after in vitro stimulation with medium alone, T. cruzi lysate, or ConA were studied in RNase protection assays. Dramatic differences in the expression of cytokine mRNA were detected in spleen cells from type 1 and type 2 biased mice after in vitro restimulation with T. cruzi lysate (Fig. 2). IFN-γ and IL-2 responses (type 1 phenotype) were increased in spleen cells harvested from mice given the type 1 bias immunization protocol. Induction of IFN-γ mRNA was increased >6-fold in type 1 compared with type 2 biased spleen cells at both 24 and 48 h (Table 2). IL-2 mRNA was increased approximately twofold at 24 h in type 1 compared with type 2 biased samples, although at 48 h there was no difference between the groups in terms of IL-2 mRNA expression. Comparably low levels of IL-4, IL-5, IL-6, and IL-13 mRNA responses (type 2 phenotype) were detected in spleen cells from both type 1 and type 2 biased samples at 24 h. However, at 48 h there were increased IL-4 (4.5-fold), IL-5 (4-fold), and IL-13 (3.4-fold) mRNA responses in type 2 compared with type 1 biased samples. IL-10 responses were similar at both 24 and 48 h in samples from type 1 and type 2 biased mice.
FIG. 2.
RNase protection assays demonstrate that the type 1 and type 2 biased intranasal vaccination protocols induced highly polarized immune responses. Total spleen cells (4 × 106 cells/ml) were harvested and rested in medium alone, stimulated with T. cruzi lysate, or stimulated with ConA, using methods similar to those described in the legend to Fig. 1. Total RNA samples were harvested from parallel cultures 24 and 48 h later using RNeasy Kits (Qiagen). These RNA samples were used in RiboQuant RNase protection assays (Pharmingen) to assess the levels of cytokine mRNA expressed. The autoradiogram shown is representative of multiple similar experiments. A progressive increase in IFN-γ mRNA expression induced by T. cruzi lysate in vitro was the most prominent finding with spleen cells from type 1 biased mice. On the other hand, parasite-specific increases in IL-4, IL-5, IL-9, and IL-13 mRNA were detected with spleen cells from type 2 biased mice, especially after 48 h of in vitro stimulation.
TABLE 2.
Differential type 1 versus type 2 induction of cytokine mRNA expressiona
| Sample | NV (ratio of cytokine to control mRNA)
|
||||||
|---|---|---|---|---|---|---|---|
| IFN-γ | IL-2 | IL-4 | IL-5 | IL-9 | IL-10 | IL-13 | |
| Type 1, 24 h | 49.3 | 13.7 | 1.7 | 1.3 | 0.91 | 1.7 | 1.5 |
| Type 2, 24 h | 7.3 | 7.4 | 2.0 | 1.5 | 0.84 | 1.6 | 2.4 |
| Type 1, 48 h | 52.9 | 3.4 | 40.0 | 0.3 | 0.97 | 2.8 | 2.0 |
| Type 2, 48 h | 7.7 | 4.2 | 180.0 | 1.2 | 2.6 | 2.5 | 6.9 |
Total splenic RNA samples were harvested from mice immunized with the T. cruzi-specific type 1 and type 2 biased vaccination protocols 24 and 48 h after in vitro stimulation with parasite lysate, as shown in Fig. 2. Densitometry was used to quantitate cytokine mRNA expression, and cytokine values were normalized for expression of the housekeeping gene L32. Normalized values (NV) were calculated as follows: NV = (cytokine densitometry measurement/L32 densitometry measurement in same lane) × 100. Shown are the results of a single experiment representative of multiple experiments with similar results.
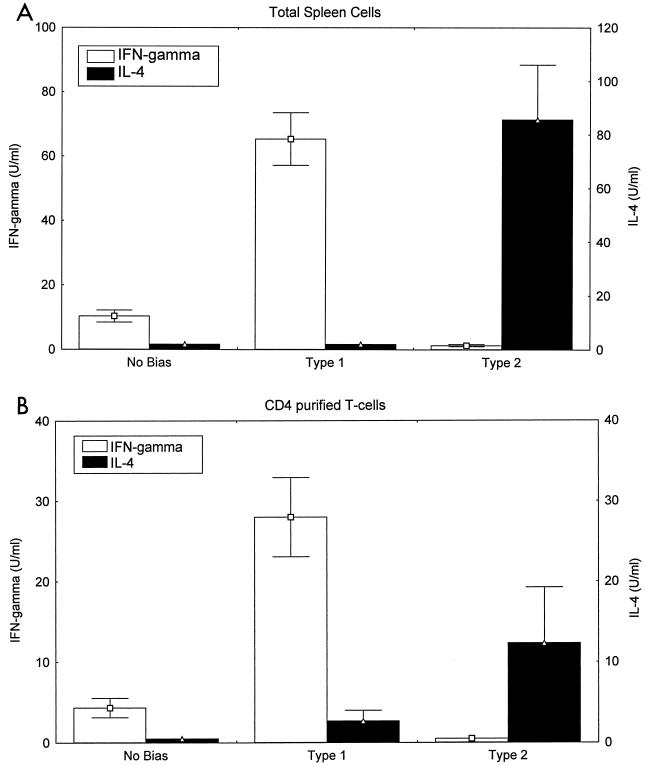
We also studied cytokine secretion by total spleen cells (Fig. 3A) and purified CD4+ T cells (Fig. 3B) from different vaccination groups after stimulation with T. cruzi lysate in vitro. Total spleen cells harvested from mice immunized with T. cruzi lysate and cholera toxin alone (nonbiased mice) produced little or no IFN-γ or IL-4 detectable in culture supernatants. Spleen cells from type 1 immunized mice produced >60 U of IFN-γ/ml and no detectable levels of IL-4. Spleen cells from type 2 immunized mice failed to produce detectable IFN-γ but did produce >80 U of IL-4/ml. Similar differential cytokine response profiles were detected in purified CD4+ splenic T cells (Fig. 3B), and with mesenteric lymph node cells and Peyer's patch lymphocytes (data not shown), from nonbiased, type 1 biased, and type 2 biased mice. T cells from naïve mice did not produce either IFN-γ or IL-4 in vitro after stimulation with T. cruzi lysate, and T cells from immunized mice did not produce measurable cytokine responses without being stimulated with parasite antigens in vitro (data not shown). Therefore, these combined cytokine RNA and protein results demonstrate that, despite similar levels of overall activation of T cells as measured by lymphoproliferative responses, the three different intranasal immunization protocols resulted in very different cytokine response profiles.
FIG. 3.
Cytokine secretion studies confirm that type 1 and type 2 biased immunization protocols induce highly polarized T. cruzi-specific immunity. BALB/c mice were immunized as described in Table 1. Three days after the final intranasal antigen challenge, mice were sacrificed and spleen cell suspensions were prepared. (A) Total spleen cells (2 × 105 cells/200 μl) were stimulated with T. cruzi lysate, and 72 h later the culture supernatants were harvested and assayed for secreted IFN-γ and IL-4 levels by ELISA. (B) Similar studies were done with purified CD4+ T cells (5 × 104 cells/200 μl) cultured with irradiated syngeneic total spleen cells (106 cells/200 μl) pulsed with T. cruzi lysate. Both sets of data had background levels of cytokines spontaneously produced in medium-rested cultures subtracted from the values presented. Shown are the means and standard errors from a single experiment with pooled cells derived from two to three mice/group. These results are representative of at least five similar experiments. Similar results were seen in lymphocytes harvested from Peyer's patches and from mesenteric lymph nodes (data not shown).
Induction of T. cruzi-specific secretory IgA responses by nonbiased, type 1 biased, and type 2 biased immunization protocols.
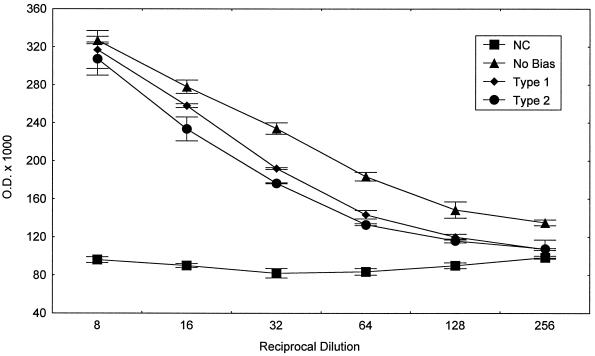
We originally hypothesized that type 2 responses would be important for the induction of optimal secretory IgA responses. We prepared fecal extracts from mice given the nonbiased, type 1 biased, and type 2 biased immunization protocols and studied the levels of T. cruzi-specific IgA present at various times from 0 to 6 weeks postvaccination. Peak responses were detected by 2 weeks after the second vaccination (data not shown). Fecal extracts from mice given cholera toxin alone intranasally and from unvaccinated mice were nonreactive with parasite lysate in this ELISA (results also not shown). Figure 4 presents the levels of T. cruzi-specific IgA detected in fecal extracts collected from negative control and immunized mice 3 days after their third immunization. Contrary to our initial hypothesis, it is clear that all three groups of mice immunized with T. cruzi lysate developed similar parasite-specific secretory IgA responses regardless of administration of additional biasing reagents. A corollary to our original hypothesis was that optimal type 1 responses might inhibit secretory IgA responses. However, our data clearly demonstrate that the type 1 bias protocol did not inhibit the overall development of T. cruzi-specific secretory IgA responses.
FIG. 4.
Nonbiased, type 1 biased, and type 2 biased intranasal vaccinations induced similar levels of T. cruzi-specific secretory IgA responses. Fecal extracts collected from mice after vaccination were studied in an IgA-specific ELISA with T. cruzi lysate. Shown are mean ELISA ODs (± the standard error) for serial dilutions in each group. Similar results were seen in multiple experiments. Calculated endpoint titers (dilution with an OD double the OD of negative controls) were between 1/30 and 1/100 for all three groups (nonbiased, type 1 biased, and type 2 biased) in all experiments. Intranasal administration of cholera toxin alone did not increase ELISA reactivity of fecal extracts with T. cruzi lysate (data not shown).
Type 1 biased intranasal immunization protects mice against lethal T. cruzi systemic challenge.
Our group has previously shown that subcutaneous vaccinations given with type 1 biasing agents protected against lethal T. cruzi systemic challenges (19), and thus we expected intranasal vaccinations given with type 1 bias to provide systemic protection as well. However, we were concerned that our mucosal type 1 vaccinations could have induced a partial systemic tolerance or could have resulted in lowered levels of systemic protection due to a selective induction of lymphocytes programmed for mucosal trafficking. Therefore, it was important to directly investigate whether or not intranasal type 1 biased vaccinations could protect against virulent systemic T. cruzi challenges. Intranasally immunized mice were challenged with 5,000 BFT subcutaneously 2 to 4 weeks after their final vaccination and followed for parasitemia and survival (Table 3). All nine unimmunized control mice developed a high parasitemia (>2 million BFT/ml) and died within 4 weeks after virulent systemic challenge. Similarly, only rarely did mice immunized with the nonbiased (two of seven survived) or the type 2 biased (one of eight survived) vaccination protocols survive the virulent systemic challenge. However, all six mice immunized with the type 1 biased intranasal protocol survived greater than 3 months postchallenge (P < 0.05), demonstrating that mucosal type 1 vaccinations can induce robust systemic protection against T. cruzi.
TABLE 3.
Systemic T. cruzi protection induced by intranasal vaccinationsa
| Immunization protocol | Survival (%)b |
|---|---|
| None | 0/9 (0) |
| Unbiased | 2/7 (29) |
| Type 1 biased | 6/6 (100)* |
| Type 2 biased | 1/8 (13) |
BALB/c mice were immunized as shown in Table 1 and challenged with 5,000 T. cruzi BFT subcutaneously in the base of the tail. Shown are the survival rates 3 months postchallenge.
*, P < 0.05 by Fisher's exact test comparing survival in type 1 immunized and all other groups.
Type 1 biased intranasal immunization provides optimal protection against mucosal T. cruzi challenge.
Type 2 responses are classically associated with helper functions for humoral immune responses, allergic responses, and immunity directed against multicellular helminthic parasites. In addition, type 2 responses have been shown to predominate in some studies of mucosal tissues and are thought to facilitate the induction of secretory IgA responses. This background knowledge led us to hypothesize that optimal mucosal protection against T. cruzi would require the optimal induction of parasite-specific type 2 responses. In order to study mucosal protection we needed to develop highly sensitive and specific methods for the measurement of T. cruzi replication at the site of initial T. cruzi mucosal invasion. Our group previously demonstrated that T. cruzi IMT exclusively infect mice through gastric epithelia after oral mucosal challenges, and we also carefully studied the kinetics of parasite dissemination from mucosal to systemic tissues after oral T. cruzi challenges (17). We found that during the first 2 weeks after oral challenge, parasites are overwhelmingly present in the gastric mucosa and draining gastric lymph nodes. Only rarely could parasites be detected in the circulating blood by either microscopic exam or PCR during this early time period after oral challenge. In addition, parallel quantitative culture and histologic studies demonstrated that the level of viable parasites recovered from mucosal tissues directly predicted the density of parasite infection detectable by immunohistochemistry. More recently, our group has developed an SQ-PCR method to quantitate T. cruzi DNA present in samples of total stomach DNA after oral T. cruzi challenges. The gene target of this SQ-PCR method is the C-terminal 418 bp of the coding region for the major T. cruzi cysteinyl proteinase, cruzipain. In the present work we compared the levels of parasite DNA (ratio of cytokine to control mRNA) at the site of initial mucosal T. cruzi invasion in vaccinated and control mice within 2 weeks after oral IMT challenge to assess the relative levels of mucosal protection provided by each vaccination protocol. As a confirmatory tool, we quantified viable parasites present in the initial draining gastric lymph nodes as previously described (17). By conducting these studies in mucosal tissues and draining lymph nodes early after oral challenges (prior to widespread dissemination of T. cruzi parasites), we can specifically and accurately assess levels of mucosal protection. The early time points studied in our present work included 6 to 8 and 10 to 14 days postchallenge (similar results were seen 1 and 2 weeks postchallenge; presented here are the 10- to 14-day studies only). Representative vaccinated and control BALB/c mice were followed for at least 2 months after oral T. cruzi challenge and no significant differences in parasitemia levels or survival were detected.
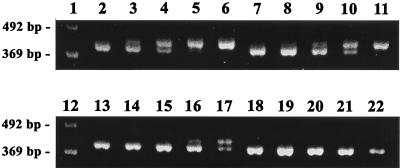
In Fig. 5, we present the sensitivity of the T. cruzi-specific SQ-PCR technique. Sets of five identical 10-fold dilutions of positive control DNA template were added to PCRs containing DNA extracted from a known number of T. cruzi parasites. Reaction products in lanes 2 to 6 were amplified with DNA samples containing 500 T. cruzi ME, products in lanes 7 to 11 were amplified with DNA samples containing 50 T. cruzi ME, products in lanes 13 to 17 were amplified with DNA samples containing 5 T. cruzi ME, and products in lanes 18 to 23 were amplified without added T. cruzi DNA. The point of equivalent intensity of positive control and wild-type cruzipain bands identifies the relative concentration of parasite equivalents present within the sample being tested. The point of equivalence shifts one lane to the right with each 10-fold decrease in T. cruzi DNA added. These results demonstrate that our SQ-PCR method can quantitate T. cruzi ME in samples over a 3 log concentration range and can detect as few as 5 T. cruzi ME.
FIG. 5.
SQ-PCR assessment of T. cruzi replication in mucosal tissues. DNA samples were extracted from mucosal tissues using QIAamp tissue kits and used as templates in PCRs with primers specific for the C-terminal 418-bp fragment of the major T. cruzi cysteinyl proteinase, cruzipain. A constant amount of each DNA sample was amplified with 10-fold dilutions of a positive control DNA fragment. The positive control contains the same 5′ and 3′ ends as the cruzipain C terminus but is smaller by 25 bp than the wild-type sequence, making it distinguishable in ethidium bromide-stained 2% TBE agarose gels. The point of equivalent band intensity for both positive control and native PCR products can be used to assess the relative quantities of T. cruzi DNA in unknown samples. Shown are four sets of five reactions with 10-fold dilutions of the positive control, each set with known samples containing 10-fold-decreasing amounts of T. cruzi DNA. Reactions in lanes 2 to 6, 7 to 11, 13 to 17, and 18 to 22 contained 500, 50, 5, and 0 parasite equivalents of T. cruzi DNA, respectively. Lanes 1 and 12 were loaded with a 123-bp ladder molecular mass standard.
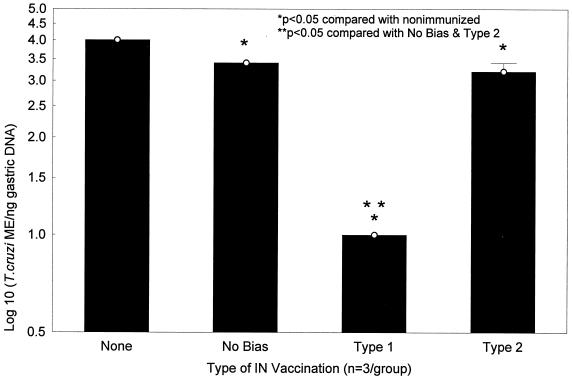
Stomach DNA samples harvested from immunized mice 10 days after oral challenge with 10,000 IMT were studied with this SQ-PCR technique (Fig. 6). All three vaccination protocols (nonbiased, type 1 biased, and type 2 biased) resulted in reduced levels of mucosal T. cruzi ME compared with levels in control mice (unimmunized or given cholera toxin alone). Stomachs harvested from nonbiased and type 2 biased mice did have significantly less T. cruzi DNA compared with stomachs harvested from unimmunized mice (<3,000 ME/ng versus >10,000 ME/ng; P < 0.05 by the Mann-Whitney U test). However, contrary to our original hypothesis, the most significantly reduced concentrations of T. cruzi DNA were detected in type 1 immunized mice (<10 ME/ng; P < 0.05 when compared with unimmunized, nonbiased immunized, and type 2 immunized mice), indicating that the type 1 vaccination protocol induced the best mucosal protection.
FIG. 6.
Type 1 responses provide optimal protection against T. cruzi mucosal infection. BALB/c mice were immunized with nonbiased, type 1 biased, and type 2 biased vaccination protocols as described in Table 1. One month after the last immunization, mice were challenged orally with 10,000 T. cruzi IMT. Ten days after oral IMT challenge, gastric DNA samples were isolated and studied by using T. cruzi-specific SQ-PCR as described in the legend to Fig. 5. Nonbiased and type 2 biased mice had lower levels of recoverable T. cruzi DNA compared with unvaccinated animals (P < 0.05 by the Mann-Whitney U test). However, type 1 biased mice were optimally protected against mucosal T. cruzi challenge, with 3 log-greater reductions in recovered parasite DNA compared with nonbiased and type 1 biased mice (P < 0.05 by the Mann-Whitney U test). Similar results were detected in multiple experiments.
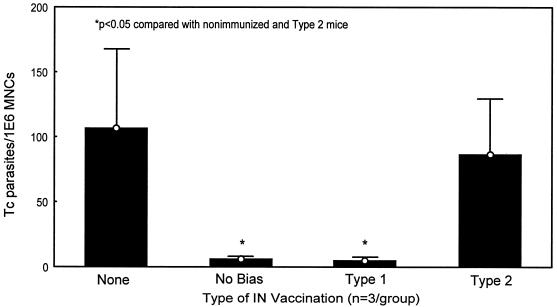
Studies of viable T. cruzi parasites detectable in draining gastric lymph nodes harvested from these same mice confirmed that optimal protective mucosal immunity was induced by the type 1 immunization protocol (Fig. 7). Viable T. cruzi parasites recoverable from draining gastric lymph nodes were significantly reduced in nonbiased and type 1 immunized mice compared with both unimmunized mice and type 2 immunized mice (<5.5 parasites/million lymph node cells versus >100 parasites/million cells; P < 0.05).
FIG. 7.
Type 1 biased immunization inhibits recovery of viable T. cruzi parasites from mucosal draining lymph nodes after oral IMT challenge. Within 1 week after delivery of oral IMT, an enlarged lymph node is visible within the lesser curvature of the stomach of challenged mice. This enlarged node represents the initial lymphatic drainage site proximal to the point of mucosal invasion. Single-cell preparations of lymphocytes from these gastric lymph nodes can be harvested and serially diluted in LDNT+ medium ideal for outgrowth of T. cruzi epimastigotes. After incubation at 26°C for 1 to 2 months, the minimal number of lymphocytes associated with detectable epimastigote outgrowth is directly proportional to the levels of replicating parasites present in the original gastric lymph node tissues. The minimal number of parasites per million lymphocytes present within the original lymph nodes is calculated by dividing 1 million by the minimal number of lymphocytes associated with parasite outgrowth. Both nonbiased and type 1 biased mice were significantly protected against T. cruzi replication in mucosal draining lymph nodes compared with unvaccinated and type 2 biased mice (P < 0.05 by the Mann-Whitney U test). Similar results were detected in multiple experiments.
DISCUSSION
Because T. cruzi can be transmitted to humans by mucosal, percutaneous, and parenteral routes, vaccines capable of inducing both mucosal and systemic parasite-specific immunity should provide optimal protection against initial T. cruzi infection. We originally hypothesized that different types of immunity (type 1 versus type 2) would be involved in the optimal protection of mucosal and systemic tissues. Based on previous work (18, 19, 23), we expected type 1 responses to be important for systemic protection mediated by intracellular microbicidal mechanisms and cytotoxic T lymphocytes. We also hypothesized that type 2 responses might be important for induction of secretory IgA or other mucosal immune responses capable of preventing or inhibiting the initial mucosal invasion by extracellular T. cruzi trypomastigotes transmitted via infected reduviid excreta. This latter projection was based on extensive previous data (reviewed in references 10 and 32), although recent observations have challenged the importance of classical type 2 responses for vaccine-induced secretory IgA production.
Studies in IL-4 knockout mice have clearly shown that IL-4-producing type 2 cells are critical for the optimal induction of secretory IgA responses by soluble protein vaccines delivered mucosally with cholera toxin (24, 30). On the other hand, IL-4 responses are not required for the induction of secretory IgA responses by attenuated salmonella or immune-stimulating complex (ISCOM)-formulated mucosal vaccines (22, 27, 29, 31). In addition, mucosal vaccinations given with IL-12 have been shown to induce both secretory IgA responses and protective immunity against systemic disease (3, 4), further evidence against the absolute importance of type 2 responses for immunity induced by mucosal vaccines. The relevance of these studies for vaccines designed to induce concurrent mucosal and systemic protection, however, is uncertain. Vaccine-induced secretory IgA responses were quantified without investigating effector function, and protection was not measured directly within mucosal tissues. The importance of type 2 responses for induction of protective secretory IgA and the relative mucosal protection provided by type 1 versus type 2 responses have not been evaluated previously.
Similar to previous reports involving other infectious pathogens (3, 4), we have shown that mucosal vaccinations given with type 1 bias can protect against systemic T. cruzi challenges. Contrary to our original hypothesis, mucosal type 1 vaccinations also provided optimal protection against T. cruzi mucosal infection. Quantitatively similar parasite-specific secretory IgA responses were induced by nonbiased, type 1 biased, and type 2 biased vaccination protocols, and mice vaccinated with the nonbiased and type 2 biased protocols were partially protected against T. cruzi mucosal infection compared with unimmunized controls. However, the most potent memory immune inhibitory effects against mucosal T. cruzi replication were detected in mice given the mucosal type 1 bias, associated with the induction of maximal IFN-γ-producing CD4+ T cells in addition to maximal secretory IgA responses. Both type 1 and secretory IgA responses may be important for optimal mucosal protection against mucosally invasive, intracellular pathogens like T. cruzi. Consistent with this interpretation, mice vaccinated with our nonbiased protocol developed intermediate IFN-γ responses (Fig. 3) in addition to maximal IgA responses (Fig. 4) and were more protected against mucosal infection than type 2 immunized mice (Fig. 7) that developed no detectable IFN-γ responses (Fig. 3) but maximal IgA responses (Fig. 4).
The enhanced partial mucosal protection provided by our nonbiased immunization protocol compared with the type 2 biased immunization protocol was evident only in the quantitative culture assays (Fig. 7) and not in the PCR assays (Fig. 6). It is likely that this discrepancy is related to the differences in sample sources studied and/or the relative specificity of the two assays for persistence of viable mucosal parasites. The quantitative cultures were done with draining gastric lymph nodes, one step removed from the superficial mucosa. The weak IFN-γ responses induced in mice vaccinated with the nonbiased protocol might have had more dramatic effects in the deeper mucosal tissues on viability of parasites than on parasite DNA levels in the superficial mucosa.
Our data have important practical implications for the development of T. cruzi vaccines. Vaccines designed to induce concurrent mucosal and systemic protection against T. cruzi can focus on the more simplified goal of inducing potent type 1 responses in both mucosal and systemic lymphoid tissues, rather than the differential induction of T-cell subsets. This more simplified approach avoids the potential problems of reciprocal inhibition between mucosal type 2 and systemic type 1 responses. In addition, a homogeneous targeting of mucosal and systemic type 1 responses will require less complex and more convenient immunization regimens than targeting heterogeneous T-cell subsets in mucosal and systemic tissues. For example, it may be possible to induce mucosal and systemic type 1 responses protective against T. cruzi with intranasal vaccines containing mixtures of parasite antigens and either CpG or liposomal IL-12 as adjuvants. Our present results have demonstrated the general feasibility of inducing concurrent mucosal and systemic protection against mucosally invasive, intracellular pathogens and provide a strong rationale for future efforts to develop vaccines designed to protect against mucosal, percutaneous, and parenteral transmission of T. cruzi infection.
As pointed out above, although type 1 responses were associated with optimal mucosal protection, the nonbiased and type 2 biased vaccination protocols provided partial protection against T. cruzi mucosal challenges and induced T. cruzi-specific secretory IgA similar to the type 1 biased protocol. These secretory IgA responses may be mechanistically involved in the partial mucosal protection induced by nonbiased and type 2 biased vaccination protocols. Further studies in B-cell knockout mice are needed to explore this hypothesis. In addition, to date we have focused on studies of vaccine-induced secretory IgA responses reactive with complex mixtures of parasite antigens present in whole T. cruzi lysates. We cannot rule out differences in either antigen specificity or functional activity of the secretory IgA responses induced by type 1 compared with less protective nonbiased and type 2 biased vaccinations. Further studies are required to investigate these possibilities as well.
In any case, our data are consistent with the salmonella and ISCOM vaccination studies cited above in showing that predominant IL-4-producing type 2 responses are not required for the induction of vaccine-specific secretory IgA responses (27, 29). IL-6, IL-10, and transforming growth factor-β produced by lymphocytes, phagocytes, and intestinal epithelial cells have been shown to compensate for the absence of classical type 2 helper effects for induction of secretory IgA production (8, 15, 31), and these alternative B-cell growth and differentiation stimuli may be involved in the induction of T. cruzi-specific secretory IgA responses induced by our type 1 biased immunization protocol. As pointed out above, previous basic immune studies demonstrating that antigen-specific secretory IgA responses can be induced in the absence of type 2 responses have not directly evaluated the relevance of these IgA responses for mucosal protection against infectious pathogens. Our results are the first to indicate that mucosal immune responses induced by alternative mechanisms not requiring predominantly IL-4-producing type 2 cells can be associated with mucosal protection against a mucosally invasive, intracellular pathogen. However, our data also indicate that secretory IgA responses alone are not sufficient for optimal T. cruzi mucosal protection. It is possible that IgA is only an immune marker of partial mucosal immunity and not a causal effector function responsible for mucosal protection.
Our present results can be considered proof of the concept that type 1 responses are important for both mucosal and systemic T. cruzi immunity. In addition, our results indicate that intranasal vaccines can induce protective type 1 and secretory IgA responses and may be ideal for inducing both protective mucosal and systemic immunity against T. cruzi and other mucosally invasive, intracellular pathogens. Several other important human pathogens establish chronic intracellular infections after mucosal invasion. The human immunodeficiency virus and Mycobacterium tuberculosis are two other examples of mucosally invasive, intracellular pathogens responsible for major worldwide epidemics of human disease and for which no highly effective vaccines are currently available. Considerable data already indicate that type 1 immune responses are important for systemic protection against human immunodeficiency virus and M. tuberculosis (reviewed in references 2 and 25). To our knowledge, the importance of type 1 versus type 2 responses for mucosal protection against these pathogens has not been studied. Future work will need to test the more generalizable hypothesis that type 1 immunity induced by intranasal vaccinations will be important for mucosal protection against these other pathogens as well.
Acknowledgments
We thank Stan Wolf (Genetics Institute) for providing recombinant murine IL-12 for these experiments.
This work was supported through NIH RO1 funding, grant no. AI-40196, awarded to D. F. Hoft.
Editor: J. M. Mansfield
REFERENCES
- 1.Abbas, A. K., K. M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature 383:787-793. [DOI] [PubMed] [Google Scholar]
- 2.Andersen, P. 2001. TB vaccines: progress and problems. Trends Immunol. 22:160-168. [DOI] [PubMed] [Google Scholar]
- 3.Arulanandam, B. P., M. O'Toole, and D. W. Metzger. 1999. Intranasal interleukin-12 is a powerful adjuvant for protective mucosal immunity. J. Infect. Dis. 180:940-949. [DOI] [PubMed] [Google Scholar]
- 4.Boyaka, P. N., M. Marinaro, R. J. Jackson, S. Menon, H. Kiyono, E. Jirillo, and J. R. McGhee. 1999. IL-12 is an effective adjuvant for induction of mucosal immunity. J. Immunol. 162:122-128. [PubMed] [Google Scholar]
- 5.Calvo Mendez, M. L., B. Nogueda Torres, and R. Alejandre Aguilar. 1992. La via oral: una puerta de acceso para Trypanosoma cruzi. Rev. Latinoam. Microbiol. 34:39-42. [PubMed] [Google Scholar]
- 6.Camargo, E. P. 1964. Growth and differentiation in Trypanosoma cruzi. I. Origin of metacyclic trypanosomes in liquid media. Rev. Inst. Med. Trop. Sao Paulo 6:93-100. [PubMed] [Google Scholar]
- 7.Chen, Y., V. J. Kuchroo, J. Inobe, D. A. Hafler, and H. L. Weiner. 1994. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science 265:1237-1240. [DOI] [PubMed] [Google Scholar]
- 8.Clyne, M., and B. Drumm. 1993. Adherence of Helicobacter pylori to primary human gastrointestinal cells. Infect. Immun. 61:4051-4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coffman, R. L., K. Varkila, P. Scott, and R. Chatelain. 1991. Role of cytokines in the differentiation of CD4+ T-cell subsets in vivo. Immunol. Rev. 123:189-207. [DOI] [PubMed] [Google Scholar]
- 10.Czerkinsky, C., F. Anjuere, J. R. McGhee, A. George-Chandy, J. Holmgren, M. P. Kieny, K. Fujiyashi, J. F. Mestecky, V. Pierrefite-Carle, C. Rask, and J. B. Sun. 1999. Mucosal immunity and tolerance: relevance to vaccine development. Immunol. Rev. 170:197-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dias, J. C. P. 1979. Mecanismos de transmissao, p. 152-174. In Z. Brener and Z. Andrade (ed.), Trypanosoma cruzi doenca de Chagas. Guanabara Koogan, Rio de Janeiro, Brazil.
- 12.Fitch, F. W., M. D. McKisic, D. W. Lancki, and T. F. Gajewski. 1993. Differential regulation of murine T lymphocyte subsets. Annu. Rev. Immunol. 11:29-48. [DOI] [PubMed] [Google Scholar]
- 13.Fukaura, H., S. C. Kent, M. J. Pietrusewicz, S. J. Khoury, H. L. Weiner, and D. A. Hafler. 1996. Induction of circulating myelin basic protein and proteolipid protein-specific transforming growth factor-β1-secreting Th3 T cells by oral administration of myelin in multiple sclerosis patients. J. Clin. Investig. 98:70-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gajewski, T. F., S. R. Schell, G. Nau, and F. W. Fitch. 1989. Regulation of T-cell activation: differences among T-cell subsets. Immunol. Rev. 111:79-110. [DOI] [PubMed] [Google Scholar]
- 15.Goodrich, M. E., and D. W. McGee. 1998. Regulation of mucosal B cell immunoglobulin secretion by intestinal epithelial cell-derived cytokines. Cytokine 10:948-955. [DOI] [PubMed] [Google Scholar]
- 16.Gus, I., M. E. Molon, and A. P. Bueno. 1993. Chagas disease. Review of 8 simultaneous cases of acute Chagas myocarditis: 25 years later. Arq. Bras. Cardiol. 60:99-101. [PubMed] [Google Scholar]
- 17.Hoft, D. F., P. L. Farrar, K. Kratz-Owens, and D. Shaffer. 1996. Gastric invasion by Trypanosoma cruzi and induction of protective mucosal immune responses. Infect. Immun. 64:3800-3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoft, D. F., R. G. Lynch, and L. V. Kirchhoff. 1993. Kinetic analysis of antigen-specific immune responses in resistant and susceptible mice during infection with Trypanosoma cruzi. J. Immunol. 151:7038-7047. [PubMed] [Google Scholar]
- 19.Hoft, D. F., A. R. Schnapp, C. S. Eickhoff, and S. T. Roodman. 2000. Involvement of CD4+ Th1 cells in systemic immunity protective against primary and secondary challenges with Trypanosoma cruzi. Infect. Immun. 68:197-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirchhoff, L. V. 2000. Trypanosoma species (American trypanosomiasis, Chagas’ disease: biology ot trypanosomes), p. 2845-2853. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 5th ed. Churchill Livingstone, Philadelphia, Pa.
- 21.Kirchhoff, L. V., S. Hieny, M. Shiver, D. Snary, and A. Sher. 1984. Cryptic epitope explains the failure of a monoclonal antibody to bind to certain isolates of Trypanosoma cruzi. J. Immunol. 133:2731-2735. [PubMed] [Google Scholar]
- 22.Kjerrulf, M., D. Grdic, M. Kopf, and N. Lycke. 1998. Induction of gut mucosal immune responses: importance of genetic background and Th1/Th2 cross-regulation. Scand. J. Immunol. 47:401-407. [DOI] [PubMed] [Google Scholar]
- 23.Kumar, S., and R. L. Tarleton. 2001. Antigen-specific Th1 but not Th2 cells provide protection from lethal Trypanosoma cruzi infection in mice. J. Immunol. 166:4596-4603. [DOI] [PubMed] [Google Scholar]
- 24.Marinaro, M., H. F. Staats, T. Hiroi, R. J. Jackson, M. Coste, P. N. Boyaka, N. Okahashi, M. Yamamoto, H. Kiyono, H. Bluethmann, K. Fujihashi, and J. R. McGhee. 1995. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J. Immunol. 155:4621-4629. [PubMed] [Google Scholar]
- 25.McMichael, A. J., and S. L. Rowland-Jones. 2001. Cellular immune responses to HIV. Nature 410:980-987. [DOI] [PubMed] [Google Scholar]
- 26.O'Garra, A. 1998. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity 8:275-283. [DOI] [PubMed] [Google Scholar]
- 27.Okahashi, N., M. Yamamoto, J. L. Vancott, S. N. Chatfield, M. Roberts, H. Bluethmann, T. Hiroi, H. Kiyono, and J. R. McGhee. 1996. Oral immunization of interleukin-4 (IL-4) knockout mice with a recombinant Salmonella strain or cholera toxin reveals that CD4+ Th2 cells producing IL-6 and IL-10 are associated with mucosal immunoglobulin A responses. Infect. Immun. 64:1516-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaw, J., R. Lainson, and H. Frahia. 1969. Consideracoes sobre a epidemiologia dos primeiros casos autocones de doenca de Chagas registrados em Belem, Para, Brasil. Rev. Saude Publica 3:153-157. [PubMed] [Google Scholar]
- 29.Smith, R. E., A. M. Donachie, F. H. McLaren, and A. M. Mowat. 1998. Preservation of mucosal and systemic adjuvant properties of ISCOMS in the absence of functional interleukin-4 or interferon-gamma. Immunology 93:556-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vajdy, M., M. H. Kosco-Vilbois, M. Kopf, G. Kohler, and N. Lycke. 1995. Impaired mucosal immune responses in interleukin 4-targeted mice. J. Exp. Med. 181:41-53. [DOI] [PubMed] [Google Scholar]
- 31.Vancott, J. L., H. F. Staats, D. W. Pascual, M. Roberts, S. N. Chatfield, M. Yamamoto, M. Coste, P. B. Carter, H. Kiyono, and J. R. McGhee. 1996. Regulation of mucosal and systemic antibody responses by T helper cell subsets, macrophages, and derived cytokines following oral immunization with live recombinant Salmonella. J. Immunol. 156:1504-1514. [PubMed] [Google Scholar]
- 32.Yamamoto, M., J. L. Vancott, N. Okahashi, M. Marinaro, H. Kiyono, K. Fujihashi, R. J. Jackson, S. N. Chatfield, H. Bluethmann, and J. R. McGhee. 1996. The role of Th1 and Th2 cells for mucosal IgA responses. Ann. N. Y. Acad. Sci. 778:64-71. [DOI] [PubMed] [Google Scholar]