Abstract
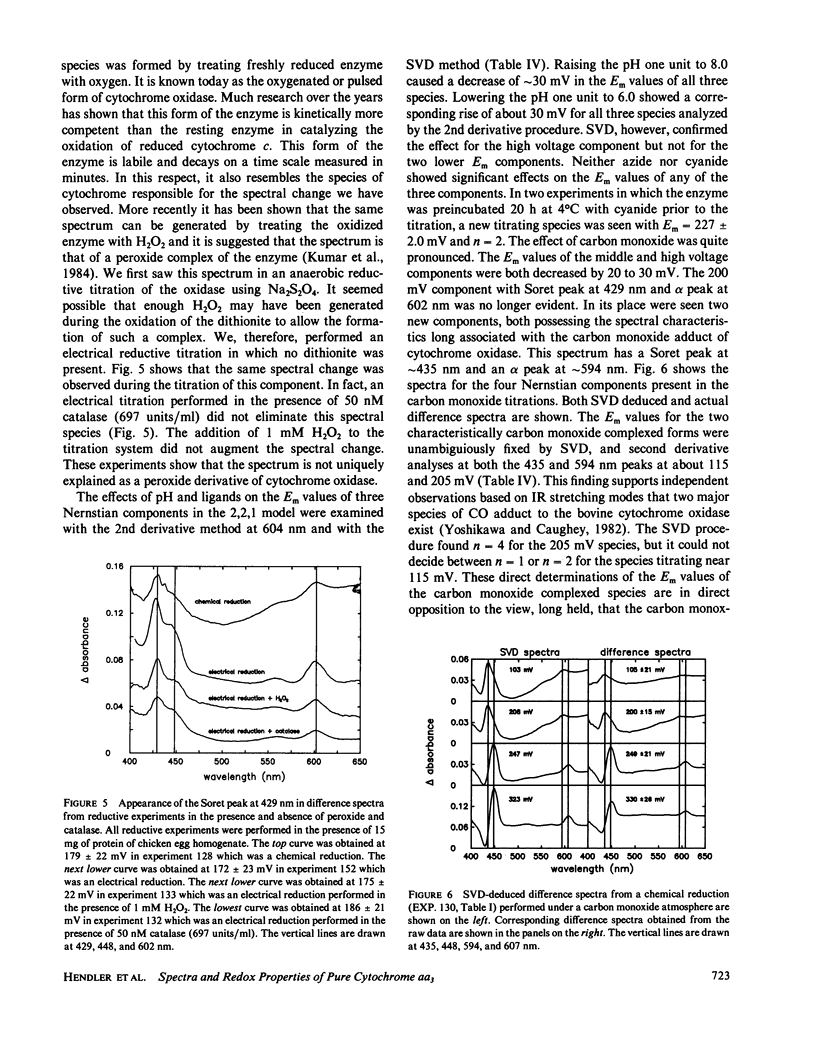
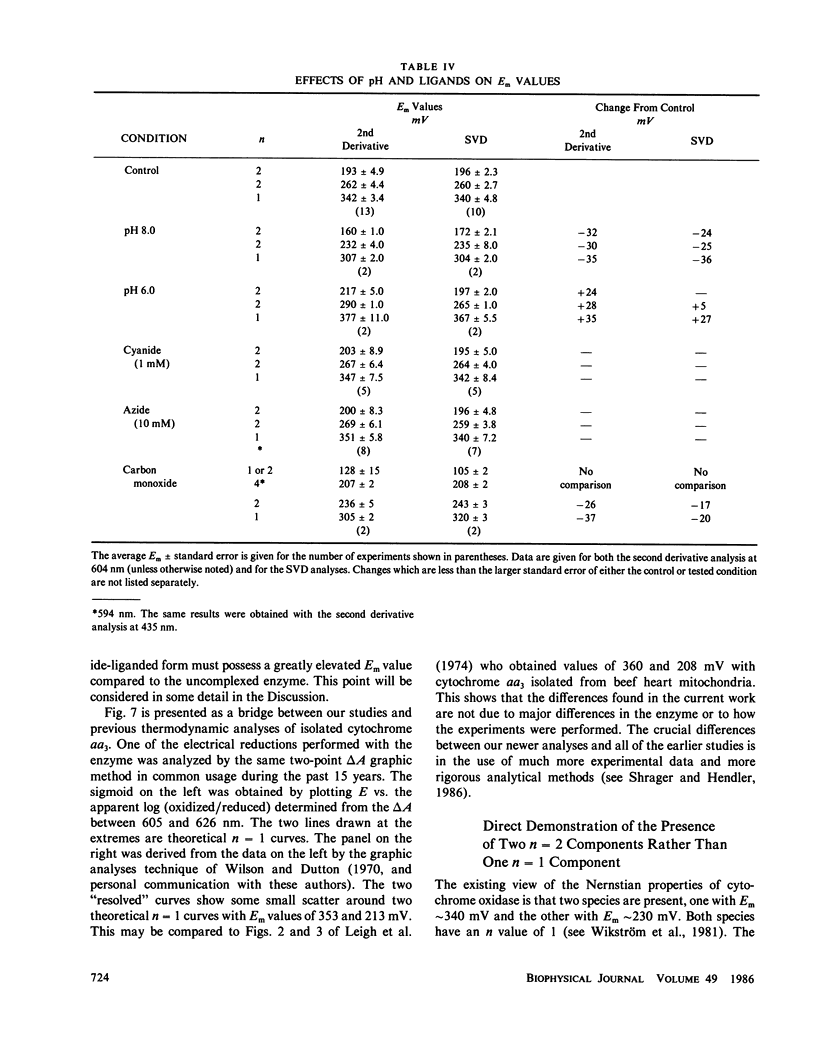
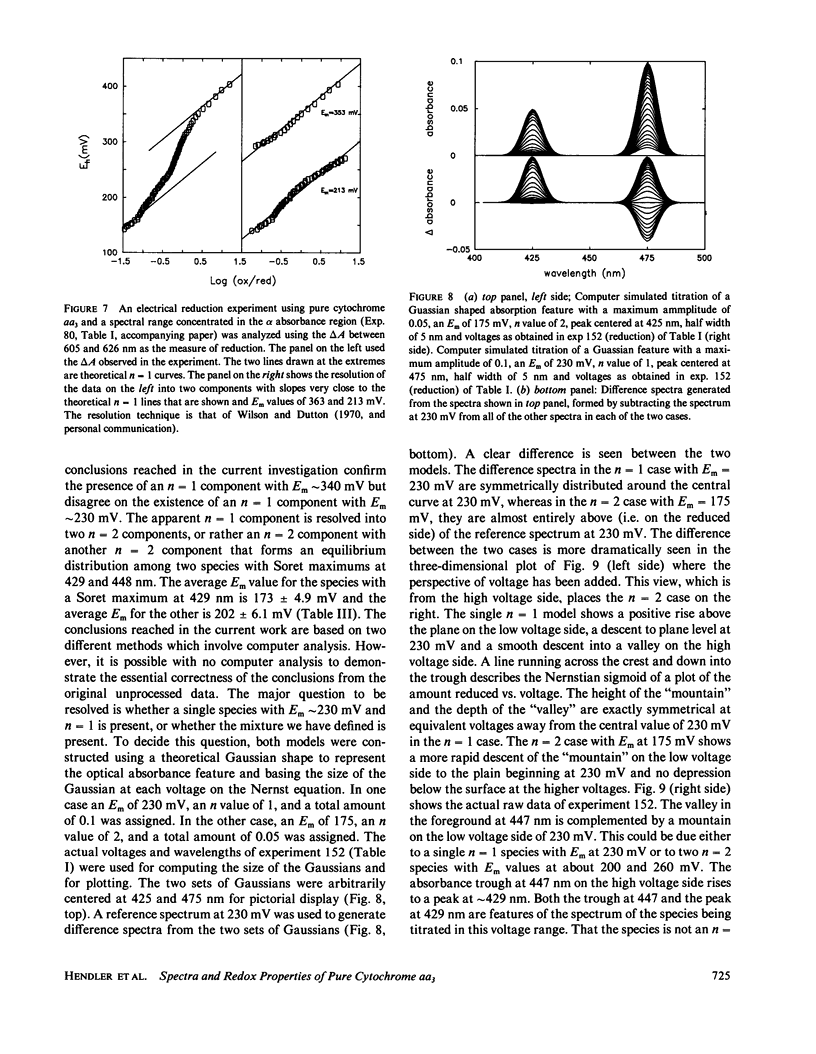
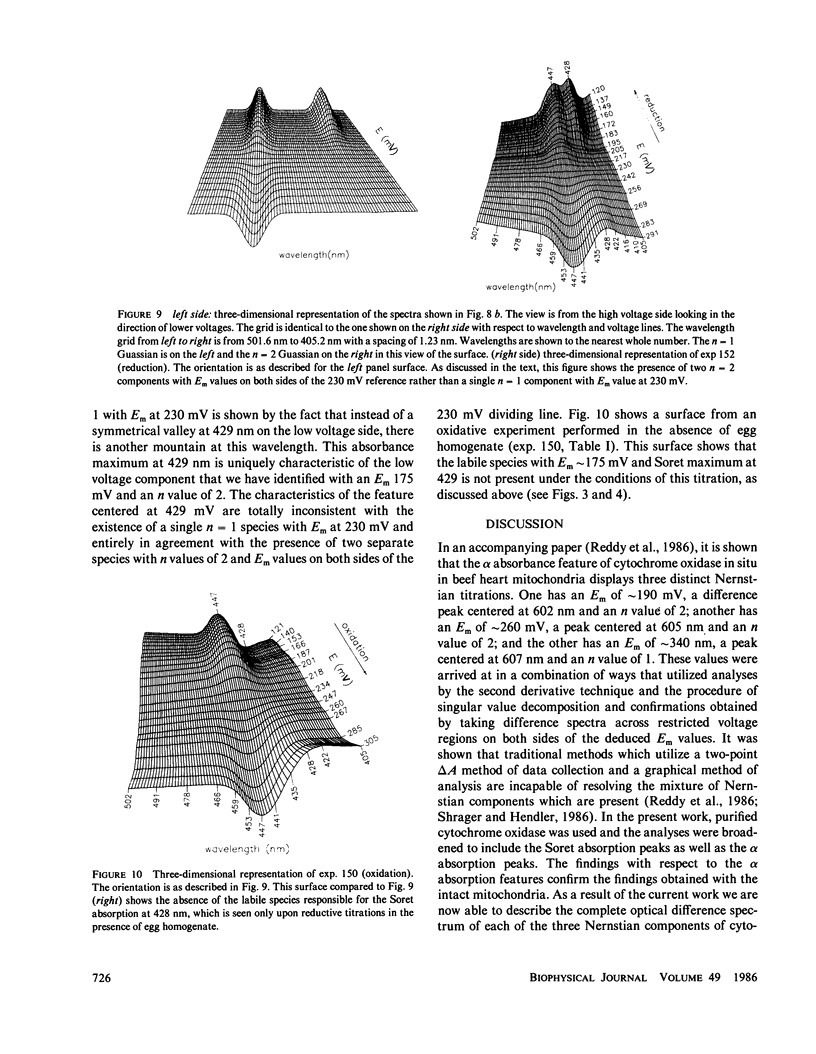
The findings in the current studies with pure cytochrome aa3 confirm the findings in an accompanying paper pertaining to cytochrome aa3 in mitochondria (Reddy et al., 1985). In both cases, three Nernstian titrations are seen with Em values near 200, 260, 340 mV with n values of 2, 2, and 1. Similarly, the alpha absorption features of the difference spectra in both cases were centered near 602, 605, and 607 mn. The component with Em approximately 200 mV was identified as heme a3 on the basis of experiments conducted in an atmosphere of carbon monoxide, and in both cases, the carbon monoxide-liganded species did not display an elevated Em. In the current studies, unique Soret absorbance features are added to the difference spectra for the three Nernstian transitions. Specifically, absorption peaks at 429, 446, and 448 nm go with the alpha peaks seen respectively at 602, 605, and 607 nm. Evidence was presented to support the hypothesis that the redox state of heme alpha may control the redox potential of heme a3.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Georgevich G., Darley-Usmar V. M., Malatesta F., Capaldi R. A. Electron transfer in monomeric forms of beef and shark heart cytochrome c oxidase. Biochemistry. 1983 Mar 15;22(6):1317–1322. doi: 10.1021/bi00275a001. [DOI] [PubMed] [Google Scholar]
- Kumar C., Naqui A., Chance B. The identity of pulsed cytochrome oxidase. J Biol Chem. 1984 Feb 25;259(4):2073–2076. [PubMed] [Google Scholar]
- Leigh J. S., Jr, Wilson D. F., Owen C. S., King T. E. Heme-heme interaction in cytochrome c oxidase: the cooperativity of the hemes of cytochrome c oxidase as evidenced in the reaction with CO. Arch Biochem Biophys. 1974 Feb;160(2):476–486. doi: 10.1016/0003-9861(74)90424-x. [DOI] [PubMed] [Google Scholar]
- Lemberg M. R. Cytochrome oxidase. Physiol Rev. 1969 Jan;49(1):48–121. doi: 10.1152/physrev.1969.49.1.48. [DOI] [PubMed] [Google Scholar]
- Nicholls P., Petersen L. C. Haem-haem interactions in cytochrome aa3 during the anaerobic-aerobic transition. Biochim Biophys Acta. 1974 Sep 20;357(3):462–467. doi: 10.1016/0005-2728(74)90038-3. [DOI] [PubMed] [Google Scholar]
- Reddy K. V., Hendler R. W., Bunow B. Complete analysis of the cytochrome components of beef heart mitochondria in terms of spectra and redox properties. Cytochromes aa3. Biophys J. 1986 Mar;49(3):705–715. doi: 10.1016/S0006-3495(86)83697-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy K. V., Hendler R. W. Complete analysis of the cytochrome components of beef heart mitochondria in terms of spectra and redox properties. The b-type cytochromes. J Biol Chem. 1983 Jul 25;258(14):8568–8581. [PubMed] [Google Scholar]
- Reddy K. V., Hendler R. W. Complete analysis of the cytochrome components of beef heart mitochondria in terms of spectra and redox properties. The c1-cytochromes. Biophys J. 1986 Mar;49(3):693–703. doi: 10.1016/S0006-3495(86)83696-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrager R. I., Hendler R. W. Processing and analysis of potentiometric data. Biophys J. 1986 Mar;49(3):687–691. doi: 10.1016/S0006-3495(86)83695-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikström K. F., Harmon H. J., Ingledew W. J., Chance B. A re-evaluation of the spectral, potentiometric and energy-linked properties of cytochrome c oxidase in mitochondria. FEBS Lett. 1976 Jun 15;65(3):259–277. doi: 10.1016/0014-5793(76)80127-5. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Dutton P. L. Energy dependent changes in the oxidation-reduction potential of cytochrome b. Biochem Biophys Res Commun. 1970 Apr 8;39(1):59–64. doi: 10.1016/0006-291x(70)90757-6. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Lindsay J. G., Brocklehurst E. S. Heme-heme interaction in cytochrome oxidase. Biochim Biophys Acta. 1972 Feb 28;256(2):277–286. doi: 10.1016/0005-2728(72)90058-8. [DOI] [PubMed] [Google Scholar]
- Yoshikawa S., Caughey W. S. Heart cytochrome c oxidase. An infrared study of effects of oxidation state on carbon monoxide binding. J Biol Chem. 1982 Jan 10;257(1):412–420. [PubMed] [Google Scholar]
- Yoshikawa S., Choc M. G., O'Toole M. C., Caughey W. S. An infrared study of CO binding to heart cytochrome c oxidase and hemoglobin A. Implications re O2 reactions. J Biol Chem. 1977 Aug 10;252(15):5498–5508. [PubMed] [Google Scholar]


