Abstract
Pathogenic spirochetes of the genus Leptospira are a major cause of human zoonotic infectious disease worldwide. After gaining entry through the skin, the organism causes disease by hematogenously disseminating to multiple organs. The mechanism by which it penetrates the mammalian cell barriers to disseminate is not well understood. In this study, we used a low-passage-number isolate of Leptospira interrogans to elucidate the invasive potential of this spirochete. Quantification of bacteria by dark-field microscopy revealed that pathogenic spirochetes were able to translocate through polarized MDCK cell monolayers at a rate significantly greater than that of nonpathogenic Leptospira or a recognized invasive bacterial pathogen, Salmonella. In contrast to Salmonella, L. interrogans did not alter transepithelial electrical resistance during cell translocation. Both transmission and scanning electron microscopy revealed tight association of the extracellular spirochetes with the host cell plasma membrane, without membrane perturbations suggestive of cytoskeletal rearrangement. Spirochetes were not observed within intercellular junctions or membrane-bound compartments inside cells. They were found within the cytoplasm of only 8% of the counted cells. These results indicate that Leptospira is an invasive but not a facultative intracellular organism. We propose that the rapid translocation of mammalian cells by pathogenic Leptospira is a mechanism designed to evade killing by host cells that permits the organism to quickly reach the bloodstream and disseminate to multiple organs.
Leptospirosis is a worldwide zoonosis caused by spirochetes belonging to the genus Leptospira (7, 21). The disease has a wide spectrum of clinical manifestations, from mild febrile illness to a severe form known as Weil's syndrome, characterized by jaundice, conjunctival suffusion, and renal failure. The hallmark of infection with Leptospira species is its rapid hematogenous dissemination after the organism gains entry into the host through abrasions in the skin. How the spirochete penetrates the mammalian cell barriers to establish disseminated infection is not known.
Experiments performed with other pathogenic spirochetes such as Treponema pallidum and Borrelia burgdorferi have provided some insight into how these helical pathogens associate with eukaryotic cells (5, 15, 37). Motile T. palladium but not nonpathogenic treponemes enter intercellular junctions of human endothelial cells (15, 37) and cross murine abdominal epithelial cell barriers within 10 h (33). B. burgdorferi was also shown to penetrate and invade both cultured tick and human umbilical vein endothelial cell (HUVEC) monolayers (5, 22, 25). Indirect immunofluorescence studies with HUVECs showed intracellular localization of Borrelia within 24 h of infection (25). These studies were confirmed by transmission electron microscopy (TEM), which provided evidence that the organisms were inside membrane-bound compartments inside cells (22).
Early in vivo experimental studies in guinea pigs support the observation of rapid hematogenous dissemination of Leptospira (6, 16). Faine reported the presence of Leptospira in the livers and kidneys of intravenously infected guinea pigs after only 30 min (6). Several reports suggest that in vitro, infection of tissue culture cells with Leptospira interrogans appears to be primarily extracellular, and adhesion to cell surfaces has been described as an important property of virulent leptospires (2, 7, 29, 38, 39). Studies have also shown that leptospires enter both phagocytic and nonphagocytic cells (26, 30, 36). In vitro, Leptospira localized free throughout the cytoplasm or in membrane-bound vesicles inside epithelial cell monolayers (30, 31, 36), while in vivo studies with experimentally infected sheep found leptospires associated with the apical plasma membrane of the kidney (27). In these studies, intact organisms were not seen invading or within cells (28). These apparently conflicting observations may be due to differences in experimental conditions and the Leptospira serovar used by various investigators.
In this study, we analyzed the interaction of a well-characterized low-passage-number patient isolate of L. interrogans with polarized cell monolayers. In an attempt to better characterize the invasive potential of this spirochete, we examined the host cell-pathogen interaction by (i) quantifying the percentage of migrating leptospires through an intact polarized cell monolayer, (ii) assessing intercellular junction integrity of the infected monolayer, (iii) characterizing cytoskeleton involvement, and (iv) visualizing L. interrogans associating with cell monolayers by both transmission and scanning electron microscopy.
MATERIALS AND METHODS
Bacterial isolates.
Leptospira organisms were cultivated in liquid Ellinghausen-McCullough-Johnson-Harris medium (Difco Laboratories, Detroit, Mich.) at 29°C and counted in a Petroff-Hausser counting chamber (Fisher Scientific, Pittsburgh, Pa). A low-passage-number clinical isolate from Brazil (21), L. interrogans serovar copenhageni strain L1-130 was used in all assays. It has a 50% lethal dose of 104 in hamsters. This strain was passaged and reisolated from hamsters twice after isolation from a blood culture of a patient with leptospirosis and stored at −70°C. Frozen aliquots were thawed and passaged in liquid medium less than two times prior to use as a low-passage-number isolate in the infection experiments. Saprophytic Leptospira biflexa Patoc 1 strain (World Health Organization Collaborative Laboratory for Leptospirosis, Royal Tropical Institute, Amsterdam, The Netherlands), Salmonella enteritidis strain 4386, and Escherichia coli DH5α were used as control bacteria in the cell monolayer assay.
Cell culture.
Madin-Darby canine kidney cells (MDCK-II) were obtained from the American Type Culture Collection (Rockville, Md.) and maintained in minimal essential medium (MEM) with sodium bicarbonate (Cellgrow; Mediatech, Herndon, Va.), supplemented with 10% fetal calf serum (Sigma, St. Louis, Mo), 5 mM l-glutamine, 100 μg of penicillin per ml, and 100 μg of streptomycin per ml. Cells were cultured in a 37°C incubator containing 5% CO2, grown to confluence in T75 flasks (Falcon, Oxnard, Calif.), and transferred to Transwell polycarbonate filters for infection assays.
Electrical measurements.
In this study, 4- and 6-day-old confluent monolayers were used. MDCK-II cells form tight junctions and polarize within a few hours under optimal conditions (13). The formation of tight junctions can be evaluated by measuring the transepithelial electrical resistance (TER) across the cell monolayer (12, 15). High electrical resistance correlates with well-developed tight junctions, while a decrease in TER indicates a disruption in the cell integrity (12). The TER of cells grown on polycarbonate filters was monitored daily with an EVOMmeter (World Precision Instruments Inc., Sarasota, Fla.) and recorded for each well before and after the infection of each cell monolayer. An average TER measurement (with standard deviation) was calculated for each time point, and each measurement was repeated in triplicate. The average TER measurement of polycarbonate filters in the absence of a cell monolayer was 90 Ω/cm2 (baseline).
Infection of polarized cell monolayers.
Epithelial cells were seeded at a density of 4 × 105 cells/cm2 in the apical side of the Transwell chamber lined with a 12-mm-diameter polycarbonate filter with a pore size of 3.0 μm (Costar Corp., Cambridge, Mass.). The chamber was placed inside another well (lower chamber) in a 12-well tissue culture plate. MDCK-II cells were washed daily with phosphate-buffered saline (PBS), pH 7.4, and replenished with fresh MEM (containing antibiotics). Spirochetes were quantified by dark-field microscopy and resuspended in l00 μl of MEM (with no antibiotics). Prior to infection, MDCK cell monolayers were washed seven times in 1× PBS (to remove any residual antibiotics) and were then infected at a multiplicity of infection (MOI) of 100 from the apical chamber. At 15, 30, 60, 120, and 240 min, 100-μl aliquots of MEM from the lower chamber were collected, and the spirochetes were quantified by dark-field microscopy.
The viability of MDCK-infected monolayers was assessed by trypan blue dye exclusion and examined by bright-field microscopy at intervals during the 15 min to 4 h of infection. Since this pathogenic strain of L. interrogans does not form distinct colonies that permit quantification by enumeration of CFU, both strains of leptospires were counted in a Petroff-Hausser chamber (25 squares counted; experiment done in triplicate) immediately following each time point. All spirochetes were visibly motile after recovery from the lower chamber. Serial dilutions of S. enteritidis and E. coli were plated at each time point (in triplicate) and quantified by enumeration of CFU. To maintain consistency of the results, we counted each well in triplicate and calculated the mean and standard deviation of the percentage of initial inoculum that had penetrated the monolayer. Means were compared with the Student's t test.
F-actin staining.
F-actin staining was performed with rhodamine-labeled phalloidin (Molecular Probes, Eugene, Oreg.). Briefly, 2 × 105 MDCK cells/cm2 were layered over round glass coverslips in 24-well tissue culture plates (Falcon). Following incubation with bacteria (15, 30, 60, and 120 min) at an MOI of 100, cell monolayers were washed three times in PBS, fixed in 2% glutaraldehyde for 10 min at room temperature, washed three times in PBS, and permeabilized with 0.1% Triton X-100 for 5 min. To minimize nonspecific binding, we incubated the cells with 1% bovine serum albumin in PBS for 30 min at room temperature and washed them three times with PBS before incubating them with rhodamine-labeled phalloidin. Slides and coverslips were mounted with Cytoseal and examined with a Zeiss fluorescence microscope.
EM.
Monolayers of Leptospira-infected MDCK-II cells were washed seven times in PBS, fixed in 2% glutaraldehyde overnight at 4°C, postfixed with 1% osmium tetraoxide and uranyl acetate, and treated with a graded series of ethanol solutions. Polycarbonate filters were cut from the Transwell apparatus and embedded in Epon 812 for TEM. The same samples were cut and prepared for critical point drying and sputter coating (12 nm) for scanning EM (SEM). The specimens for TEM were examined with the FEI Tecnai 12 electron microscope, while those for SEM were examined with the Hitachi S-5000 cold field emission SEM.
RESULTS
Rapid translocation of polarized MDCK cell monolayers.
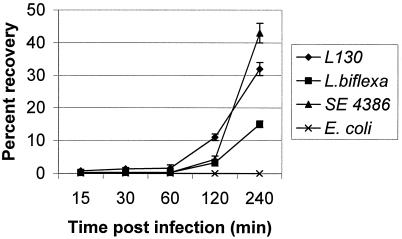
After apical infection of MDCK cells, pathogenic leptospires were observed in the lower chamber of the Transwell apparatus as early as 15 min postinfection. Of the initial inoculum, 1.6% ± 0.2% of leptospires were found in the lower chamber between 15 to 60 min (Fig. 1), while only 0.3% ± 0.04% of the invasive Salmonella strain was recovered from the lower chamber during the same time interval (P < 0.005). Moreover, both pathogenic and nonpathogenic leptospires were able to penetrate epithelial cell monolayers, but a greater percentage of the inoculum of the pathogenic L. interrogans serovar copenhageni crossed the epithelial cell barrier compared to the saprophytic L. biflexa Patoc 1. Both pathogenic and nonpathogenic leptospires crossed the polycarbonate filters at the same rate in the absence of cell monolayers (data not shown). By 4 h postinfection, 32% ± 3% of the pathogenic strain had crossed the cell membrane, while only 15% ± 2% of the saprophytic strain penetrated the monolayer (P < 0.01). Similarly, by 4 h, 43% of the inoculum of the invasive strain of S. enteritidis were recovered from the lower chamber. As expected, no organisms were recovered from the lower chamber in MDCK cell monolayers infected with a laboratory strain of E. coli.
FIG. 1.
Percent recovery of L. interrogans serovar copenhageni L1-130 (L130), L. biflexa Patoc 1, S. enteritidis strain 4386 (SE 4386), and E. coli strain DH5α after penetration of polarized MDCK cell monolayers between 15 and 240 min postinfection. Each point is the mean percent recovery ± standard deviation; each assay was done in triplicate for each organism. We counted 25 squares to enumerate the organisms in the lower chamber (experiment done in triplicate).
Leptospira spp. do not affect TER of MDCK cell monolayers.
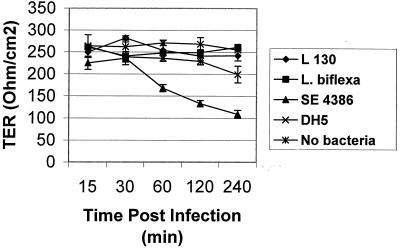
The integrity of tight junctions of the infected MDCK cell monolayers was assessed by TER measurements (Fig. 2). TER measurements of MDCK cell monolayers were taken before and at each postinfection time period of 15, 30, 60, 120, and 240 min. After infection with Leptospira, only a minor decrease in the TER was observed after 4 h. During the first 60 min, TER decreased by approximately 10%. At 120 and 240 min, TER measurements were seen to decrease by 12 and 16%, respectively. Similarly, the TER measurements of uninfected cells changed minimally between 120 and 240 min. However, TER measurements of cells infected with S. enteritidis decreased by 19% at 15 min and by 63% at 240 min. These data suggest that Leptospira organisms penetrate and translocate polarized cell monolayers without greatly disrupting cell junction integrity.
FIG. 2.
Change in TER of polarized MDCK cells during 15 to 240 min of apical infection with L. interrogans serovar copenhageni L1-130 (L 130), L. biflexa Patoc 1, S. enteritidis strain 4386 (SE 4386), and E. coli strain DH5α (DH5). Each point is the mean TER decrease ± standard deviation. Each assay was done in triplicate. The baseline value for a filter with no bacteria was 98 Ω/cm2.
Leptospira penetration of MDCK cells does not rely on actin cytoskeletal rearrangements.
The distribution of rhodamine-labeled phalloidin revealed no evidence of actin cytoskeleton condensation during 4 h of coincubation with pathogenic leptospires (data not shown).
L. interrogans was found closely associated with microvilli and inside MDCK cells.
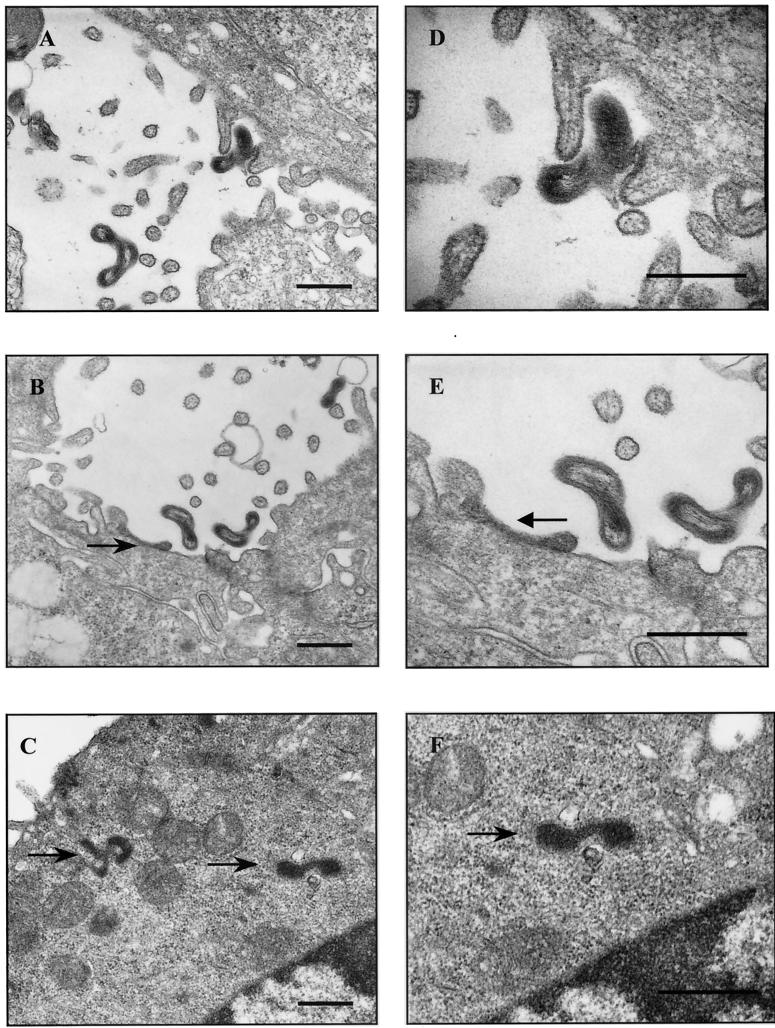
Examination of transmission electron micrographs of leptospire-infected MDCK cell monolayers demonstrated organisms both closely associated with and inside the cell as early as 30 min postinfection. Of the 50 polarized cells examined by TEM, many of the spirochetes were seen in association with and entering the cell membrane (Fig. 3A and D) and in tight association with microvilli of the apical cell surface (Fig. 3B and E), but none were seen in the intercellular spaces. There appears to be no clear staining of the cell plasma membrane at the site of entry of the organism (Fig. 3A). There was no evidence of pedestal or filopodium projections at the site of attachment of the spirochetes. Leptospires were also seen inside the cell cytoplasm (Fig. 3C and F). However, of the 50 cells examined, only 4 (8%) contained any intracellular organisms, and each infected cell appeared to contain only one organism. Moreover, there was no evidence that these organisms were inside a membrane-bound vacuole (Fig. 3F). Intercellular junctions appeared to retain integrity throughout the time course of infection.
FIG. 3.
High- and low-magnification transmission electron micrographs of L. interrogans-infected MDCK cells. (A and D) Spirochete L. interrogans, 30 min postinfection, entering the cell membrane; (B and E) L. interrogans in tight association with the plasma membrane 30 min postinfection; (C and F) L. interrogans, free inside the cell cytoplasm 60 min postinfection. Arrows point to L. interrogans. Bars, 200 μm
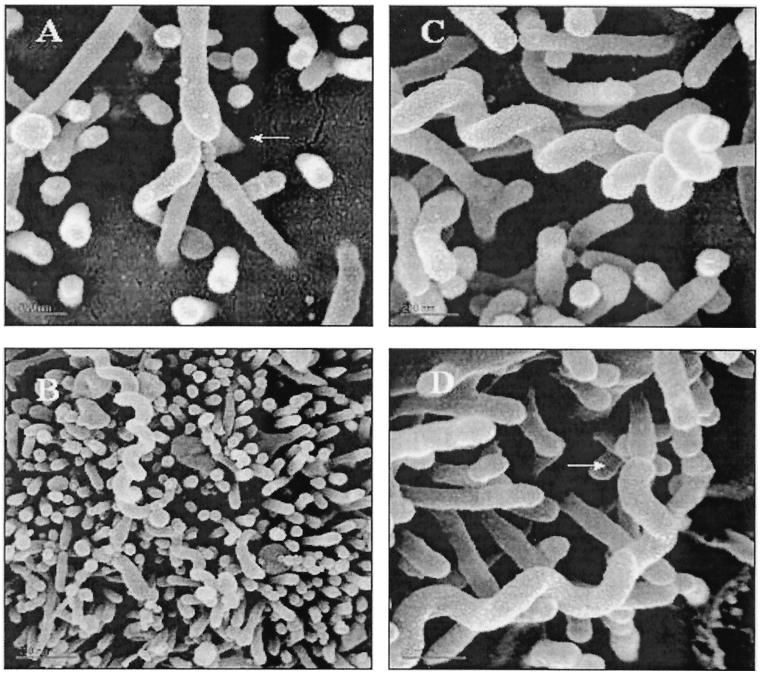
Scanning electron micrographs revealed L. interrogans entering (Fig. 4A) or in close association with microvilli on the apical surface of the monolayer with little disruption or perturbation of the epithelial cell surface (Fig. 4B to D). Moreover, there was no evidence of surface epithelial cell damage attributable to the presence of the spirochetes.
FIG. 4.
Scanning electron micrographs of L. interrogans (arrow) penetrating the MDCK cell monolayer 30 min postinfection (A) and in tight association with microvilli and the cell membrane (B to D). Bars, 200 μm.
DISCUSSION
The above observations reveal a pattern of interaction with mammalian cells of pathogenic Leptospira that is quite distinct from other well-recognized invasive bacterial pathogens, such as Salmonella, Yersinia, or Shigella spp. These latter groups of organisms usually establish invasive intestinal infections after oral inoculation. Salmonella and Yersinia organisms may disseminate via the bloodstream. Patients who develop severe leptospirosis acquire the infection through exposure to an environment contaminated with animal urine containing Leptospira (23). In places where leptospirosis is endemic like Brazil, the sewer rat, Rattus norvegicus, is the major reservoir for L. interrogans serovar copenhageni (21). The organism is thought to penetrate the skin or breaks in the skin to initiate infection and then rapidly disseminates via the bloodstream to cause multisystem infection, targeting the liver and kidney. In rats, infection results in chronic colonization of the proximal renal tubules, from which the organism is shed in the urine, thus facilitating transmission to other hosts (7).
The ability of L. interrogans to cause such a rapid systemic infection after penetration of the skin suggests that it is a highly invasive pathogen. Use of the Transwell chemotaxis chambers allowed us to demonstrate that 1.6% of the initial inoculum of pathogenic leptospires crossed polarized monolayers within 15 min of infection and that after 4 h, 32% of the inoculum of L. interrogans was found to have translocated the cellular barrier. Similar results were described in other reports (30, 36), although in our experiments, both pathogenic and nonpathogenic strains were recovered after 4 h. Hence, the ability to cross the monolayer per se does not appear to be a phenotype associated with pathogenicity. The pathogenic strain exhibited significantly faster translocation ability than the nonpathogenic strain (Fig. 1), which suggests that this ability of Leptospira to rapidly translocate through the cells may be the distinguishing feature of its pathogenicity.
It has been well established that spirochetes such as T. pallidum and B. burgdorferi can penetrate and cross cell monolayers. The current paradigm is that these pathogenic organisms disseminate through the interjunctional spaces or through the cell membrane (5, 15, 37). However, studies with leptospires have provided conflicting reports made by both in vitro and in vivo observations regarding the cellular localization (extracellular, phagosomal, cytoplasmic, or interjunctional) of Leptospira in nonprofessional phagocytic cells (2, 5, 26-28, 30, 31, 34, 36, 38). These conflicting observations may relate to differences in experimental conditions used, such as differences in the Leptospira serotypes, tissue culture cell types, and animal models.
Merien et al. (30) compared the abilities of pathogenic (L. interrogans serovar icterohemorrhagiae) and nonpathogenic leptospires (L. biflexa) to be internalized into both Vero and macrophage J774 cells. Unlike our results, they observed that only virulent leptospires were internalized, whereas saprophytic and avirulent strains remained extracellular (30). They also postulated that invasion of epithelial cells may be a way for the organism to escape the host immune response, although our results indicate that very few leptospires reside inside the cell at any time point. The intracellular organisms we saw may simply represent spirochetes in transit at the time of cell fixation for the TEM analysis. There was no evidence for intracellular multiplication, escape from membrane-bound compartments, or cell-to-cell spread of the organism, characteristics of some facultative intracellular bacterial pathogens.
When facultative intracellular bacterial organisms such as Salmonella and Shigella enter nonphagocytic mammalian cells, they characteristically induce cytoskeletal rearrangement associated with plasma membrane perturbations that lead to phagocytosis of the organism (3, 10, 32, 40). Organisms such as Yersinia spp. enter nonphagocytic cells by a receptor-mediated process, sometimes referred to as the “zipper” mechanism, in which the bacteria express a surface ligand that binds to a specific mammalian receptor in a process that allows the cell's plasma membrane to tightly surround the surface of the entire organism (8, 17, 18). We did observe tight association of the plasma membrane with the bacterial surface, resembling the “intimate attachment” seen with organisms such as enteropathogenic E. coli (11, 20) (Fig. 3B and E). Interestingly, however, despite the above observation, there was no evidence by transmission electron or fluorescence microscopy of any cell membrane perturbations or evidence of cytoskeleton rearrangement elicited by Leptospira. These results are in accordance with reported observations that cytochalasin D, an organic fungal compound that irreversibly binds actin and blocks actin polymerization, had no inhibitory effect on the internalization of leptospires (30). Moreover, TEM revealed no evidence of plasma membrane perturbations suggestive of macropinocytosis or “ruffling” reported with Salmonella epithelial cell invasion (3, 9, 19, 40). Clearly, the organism could be seen inside cells (Fig. 3C), suggesting that it entered the lower chamber of the polarized monolayer by translocating through the cells. It is also possible that the organism crossed the monolayer through interjunctional spaces, but we did not observe any organism in such spaces by TEM. Furthermore, infection by Leptospira of MDCK cells had little effect on the monolayer integrity, as previously reported with T. pallidum (37). TER decreased only 16% over the course of 4 h in contrast to 63% with Salmonella infection.
The ultimate productive outcome of pathogenic leptospires may be the complete cell translocation. In vivo, this would facilitate rapid entry into and out of the bloodstream to infect target organs, such as the kidneys (1). Thus, Leptospira may be an invasive but not a facultative intracellular pathogen. We therefore propose that Leptospira organisms invade cells but escape them rapidly to avoid intracellular killing. How Leptospira organisms achieve this high-speed cell translocation is not known. The characteristic helical morphology may play an important role in their movement through the environment (14, 24), as seen by their ability to bore through highly viscous gel-like media, such as connective tissues, which inhibit the motility of most other bacteria (4). Motility itself as a virulence factor was examined in B. burgdorferi, where a nonmotile mutant with a markedly hindered ability to penetrate HUVEC monolayers was found (35). With Leptospira, the ability to pass through cells may not be as important as the rate at which they penetrate them. This may facilitate rapid dissemination, before cell barriers or circulating immune cells can inhibit them. This rapid cell translocation phenotype of Leptospira may be a characteristic feature of pathogenic members of this spirochete.
Acknowledgments
We thank Patrick Killoran for help in preparing samples for TEM and Gordon Vrdoljak and Reena Zalpuri at the UC Berkeley Electron Microscopy Laboratory for technical assistance. We also thank Brendan Flannery for helpful comments on the manuscript.
This work was supported in part by the Oswaldo Cruz Foundation/Brazilian Ministry of Health (Biomanguinhos 09224-7), the Brazilian National Research Council (52.1229/98-7, 30.0861/96-6, 35.0052/95-6, and FINEP 4196086200), the Fogarty Program in International Research and Training in Emerging Infectious Diseases (TW00905 and TW00919), and a KO8 award from the National Institutes of Allergy and Infectious Diseases (grant AI01605).
Editor: B. B. Finlay
REFERENCES
- 1.Arean, V. M. 1962. The pathologic anatomy and pathogenesis of fatal human leptospirosis (Weil's disease). Am. J. Pathol. 40:393-423. [PMC free article] [PubMed] [Google Scholar]
- 2.Ballard, S. A., M. Williamson, B. Adler, T. Vinh, and S. Faine. 1986. Interactions of virulent and avirulent leptospires with primary cultures of renal epithelial cells. J. Med. Microbiol. 21:59-67. [DOI] [PubMed] [Google Scholar]
- 3.Brumell, J. H., O. Steele-Mortimer, and B. B. Finlay. 1999. Bacterial invasion: force feeding by Salmonella. Curr. Biol. 9:R277-R280. [DOI] [PubMed] [Google Scholar]
- 4.Chunhao, L., M. A. Motaleb, M. Sal, S. F. Goldstein, and N. W. Charon. 2000. Spirochete periplasmic flagella and motility. J. Mol. Microbiol. Biotechnol. 2:345-354. [PubMed] [Google Scholar]
- 5.Comstock, L. E., and D. D. Thomas. 1989. Penetration of endothelial cell monolayers by Borrelia burgdorferi. Infect. Immun. 57:1626-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faine, S. 1957. Virulence in Leptospira. I. Reactions of guinea-pigs to experimental infection with Leptospira icterohaemorrhagiae. Br. J. Exp. Pathol. 37:1-7. [PMC free article] [PubMed] [Google Scholar]
- 7.Faine, S., B. Adler, C. Bolin, and P. Perolat. 1999. Leptospira and leptospirosis, 2nd ed. MediScie, Melbourne, Australia.
- 8.Falkow, S., R. R. Isberg, and D. A. Portnoy. 1992. The interaction of bacteria with mammalian cells. Annu. Rev. Cell Biol. 8:333-363. [DOI] [PubMed] [Google Scholar]
- 9.Finlay, B. B. 1994. Cell biology of Salmonella pathogenesis, p. 249-261. In V. L. Miller, J. B. Kaper, D. A. Portnoy, and R. R. Isberg (ed.), Molecular genetics of bacterial pathogenesis. ASM Press, Washington, D.C.
- 10.Finlay, B. B., B. Gumbiner, and S. Falkow. 1988. Penetration of Salmonella through a polarized Madin-Darby canine kidney epithelial cell monolayer. J. Cell Biol. 107:221-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finlay, B. B., I. Rosenshine, M. S. Donnenberg, and J. B. Kaper. 1992. Cytoskeletal composition of attaching and effacing lesions associated with enteropathogenic Escherichia coli adherence to HeLa cells. Infect. Immun. 60:2541-2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez-Mariscal, L., B. Chavez de Ramirez, and M. Cereijido. 1985. Tight junction formation in cultured epithelial cells (MDCK). J. Membr. Biol. 86:113-125. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Mariscal, L., R. G. Contreras, J. J. Bolivar, A. Ponce, B. Chavez de Ramirez, and M. Cereijido. 1990. The role of calcium in tight junction formation between epithelial cells. Am. J. Physiol. 259:C978-C986. [DOI] [PubMed] [Google Scholar]
- 14.Greenberg, E. P., and E. Canale-Parola. 1977. Relationship between cell coiling and motility of spirochetes in viscous environments. J. Bacteriol. 131:960-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haake, D. A., and M. A. Lovett. 1994. Interjunctional invasion of endothelial cell monolayers. Methods Enzymol. 236:447-463. [DOI] [PubMed] [Google Scholar]
- 16.Inada, R., Y. Ido, R. Hoki, R. Kaneko, and H. Ito. 1917. The etiology, mode of infection, and specific therapy of Weil's disease (Spirochaetosis Icterohaemorrhagica). J. Exp. Med. 13:377-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isberg, R. R., and S. Falkow. 1985. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature 317:262-264. [DOI] [PubMed] [Google Scholar]
- 18.Isberg, R. R., D. L. Voorhis, and S. Falkow. 1987. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell 60:861-871. [DOI] [PubMed] [Google Scholar]
- 19.Jepson, M. A., C. B. Collares-Buzato, M. A. Clark, B. H. Hirst, and N. L. Simmons. 1995. Rapid disruption of epithelial barrier function by Salmonella typhimurium is associated with structural modification of intercellular junctions. Infect. Immun. 63:356-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knutton, S., D. R. Lloyd, and A. S. McNeish. 1987. Adhesion of enteropathogenic Escherichia coli to human intestinal enterocytes and cultured human intestinal mucosa. Infect. Immun. 55:69-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ko, A. I., M. G. Reis, C. R. Dourado, W. D. Johnson, L. W. Riley, and the Salvador Leptospirosis Study Group. 1999. Urban epidemic of severe leptospirosis in Brazil. Lancet 354:820-825. [DOI] [PubMed] [Google Scholar]
- 22.Kurtti, T. J., U. G. Munderloh, D. E. Krueger, R. C. Johnson, and T. G. Schwan. 1993. Adhesion to and invasion of cultured tick (Acarina: Ixodidae) cells by Borrelia burgdorferi (Spirochaetales: Spirochaetaceae) and maintenance of infectivity. J. Med. Entomol. 30:586-596. [DOI] [PubMed] [Google Scholar]
- 23.Lovett, P. N. 2001. Leptospirosis. Clin. Microbiol. Rev. 14:296-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lux, R., J. N. Miller, N. Park, and W. Shi. 2001. Motility and chemotaxis in tissue penetration of oral epithelial cell layers by Treponema denticola. Infect. Immun. 69:6276-6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma, Y., A. Sturrock, and J. J. Weis. 1991. Intracellular localization of Borrelia burgdorferi within human endothelial cells. Infect. Immun. 59:671-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marangoni, A., R. Aldini, V. Sambri, M. Montagnani, G. Ballardini, E. Storni, and R. Cevenini. 2000. Uptake and killing of Leptospira interrogans and Borrelia burgdorferi, spirochetes pathogenic to humans, by reticuloendothelial cells in perfused rat liver. Infect. Immun. 68:5408-5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marshall, R. B. 1976. The route of entry of leptospires into the kidney tubule. J. Med. Microbiol. 9:149-154. [DOI] [PubMed] [Google Scholar]
- 28.Marshall, R. B. 1974. Ultrastructural changes in renal tubules of sheep following experimental infection with Leptospira interrogans serotype Pomona. J. Med. Microbiol. 7:505-508. [DOI] [PubMed] [Google Scholar]
- 29.Merien, F., J. Truccolo, G. Baranton, and P. Perolat. 2000. Identification of a 36-kDa fibronectin-binding protein expressed by a virulent variant of Leptospira interrogans serovar icterohaemorrhagiae. FEMS Microbiol. Lett. 185:17-22. [DOI] [PubMed] [Google Scholar]
- 30.Merien, F., G. Baranton, and P. Perolat. 1997. Invasion of Vero cells and induction of apoptosis in macrophages by pathogenic Leptospira interrogans are correlated with virulence. Infect. Immun. 65:729-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller, N. G., and R. B. Wilson. 1962. In vivo and in vitro observations of Leptospira pomona by electron microscopy. J. Bacteriol. 84:569-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pace, J., M. J. Hayman, and J. E. Galán. 1993. Signal transduction and invasion of epithelial cells by Salmonella typhimurium. Cell 72:505-514. [DOI] [PubMed] [Google Scholar]
- 33.Riviere, G. R., D. D. Thomas, and C. M. Cobb. 1989. In vitro model of Treponema pallidum invasiveness. Infect. Immun. 57:2267-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rose, G. W., W. C. Eveland, and H. C. Ellinghausen. 1966. Mechanisms of tissue cell penetration by Leptospira pomona active penetration studies in vitro. Am. J. Vet. Res. 27:1461-1471. [PubMed] [Google Scholar]
- 35.Sadziene, A., D. D. Thomas, V. G. Bundoc, S. C. Holt, and A. G. Borbour. 1991. A flagella-less mutant of Borrelia burgdorferi. Structural, molecular, and in vitro functional characterization. J. Clin. Investig. 88:82-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas, D. D., and L. M. Higbie. 1990. In vitro association of Leptospira with host cells. Infect. Immun. 58:581-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas, D. D., M. Navab, D. A. Haake, A. M. Fogelman, J. N. Miller, and M. A. Lovett. 1988. Treponema pallidum invades intercellular junctions of endothelial cell monolayers. Proc. Natl. Acad. Sci. USA 85:3608-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsuchimoto, M., M. Niikura, E. Ono, H. Kida, and R. Yanagawa. 1984. Leptospiral attachment to cultured cells. Zentbl. Bakteriol. Mikrobiol. Hyg. 258:268-274. [DOI] [PubMed] [Google Scholar]
- 39.Vinh, T., S. Faine, and B. Adler. 1984. Adhesion of leptospires to mouse fibroblasts (L929) and its enhancement by specific antibody. J. Med. Microbiol. 18:73-85. [DOI] [PubMed] [Google Scholar]
- 40.Zhou, D., M. S. Mooseker, and J. E. Galán. 1999. An invasion-associated Salmonella protein modulates the actin-bundling activity of plastin. Proc. Natl. Acad. Sci. USA 96:10176-10181. [DOI] [PMC free article] [PubMed] [Google Scholar]