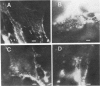
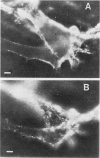
Abstract
Scanning fluorescence correlation spectroscopy is a new approach to measuring changes in the state of aggregation of cell membrane proteins. Measurements of the mean number of aggregates of virus glycoproteins from Sindbis virus and vesicular stomatitis virus agree with the findings of a recent fluorescence photobleaching recovery study on the same systems (Johnson, D.C., M.J. Schlesinger, and E.L. Elson, 1981, Cell, 23:423-431). Sindbis Virus glycoproteins are immobilized and cannot be induced to aggregate further by antibody cross linking. In this study, we find that Sindbis virus glycoprotein is more highly aggregated than vesicular stomatitis virus glycoprotein, which can be patched further with antibody. These measurements demonstrate the potential of scanning fluorescence correlation spectroscopy in studies of aggregation problems in membranes of cultured cells.
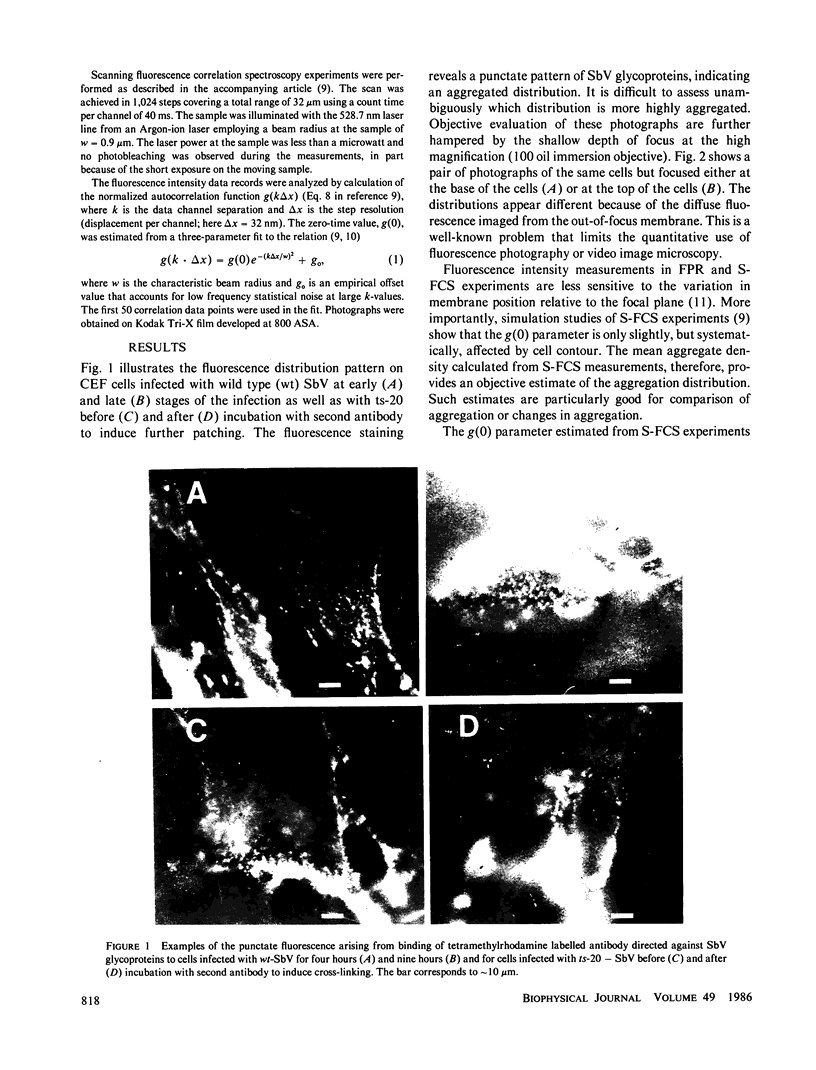
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Elson E. L., Webb W. W. Concentration correlation spectroscopy: a new biophysical probe based on occupation number fluctuations. Annu Rev Biophys Bioeng. 1975;4(00):311–334. doi: 10.1146/annurev.bb.04.060175.001523. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Schlesinger M. J., Elson E. L. Fluorescence photobleaching recovery measurements reveal differences in envelopment of Sindbis and vesicular stomatitis viruses. Cell. 1981 Feb;23(2):423–431. doi: 10.1016/0092-8674(81)90137-9. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Schlesinger M. J. Vesicular stomatitis virus and sindbis virus glycoprotein transport to the cell surface is inhibited by ionophores. Virology. 1980 Jun;103(2):407–424. doi: 10.1016/0042-6822(80)90200-7. [DOI] [PubMed] [Google Scholar]
- Lenard J., Compans R. W. The membrane structure of lipid-containing viruses. Biochim Biophys Acta. 1974 Apr 8;344(1):51–94. doi: 10.1016/0304-4157(74)90008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magde D., Elson E. L., Webb W. W. Fluorescence correlation spectroscopy. II. An experimental realization. Biopolymers. 1974 Jan;13(1):29–61. doi: 10.1002/bip.1974.360130103. [DOI] [PubMed] [Google Scholar]
- Petersen N. O. Diffusion and aggregation in biological membranes. Can J Biochem Cell Biol. 1984 Nov;62(11):1158–1166. doi: 10.1139/o84-149. [DOI] [PubMed] [Google Scholar]
- Petersen N. O., McConnaughey W. B. Effects of multiple membranes on measurements of cell surface dynamics by fluorescence photobleaching. J Supramol Struct Cell Biochem. 1981;17(3):213–221. doi: 10.1002/jsscb.380170303. [DOI] [PubMed] [Google Scholar]
- Petersen N. O. Scanning fluorescence correlation spectroscopy. I. Theory and simulation of aggregation measurements. Biophys J. 1986 Apr;49(4):809–815. doi: 10.1016/S0006-3495(86)83709-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidler J. A., Keller P. M., Elson E. L., Lenard J. A fluorescence photobleaching study of vesicular stomatitis virus infected BHK cells. Modulation of G protein mobility by M protein. Biochemistry. 1981 Mar 3;20(5):1345–1349. doi: 10.1021/bi00508a047. [DOI] [PubMed] [Google Scholar]