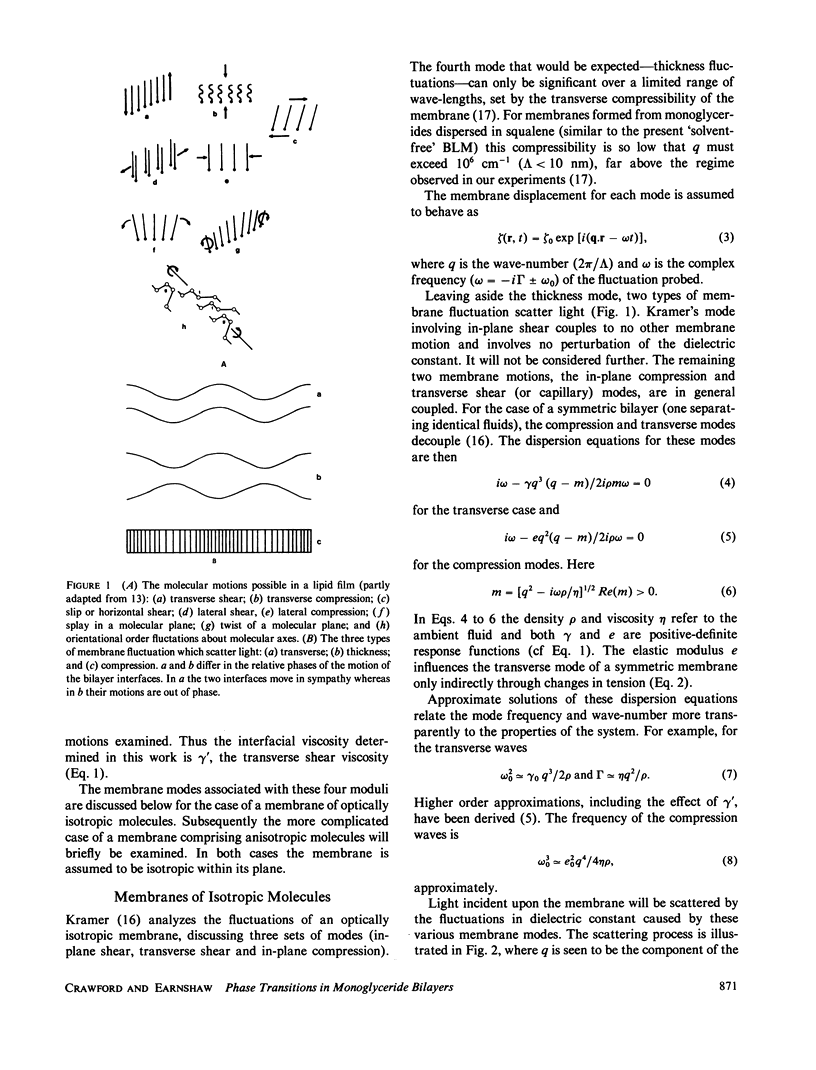
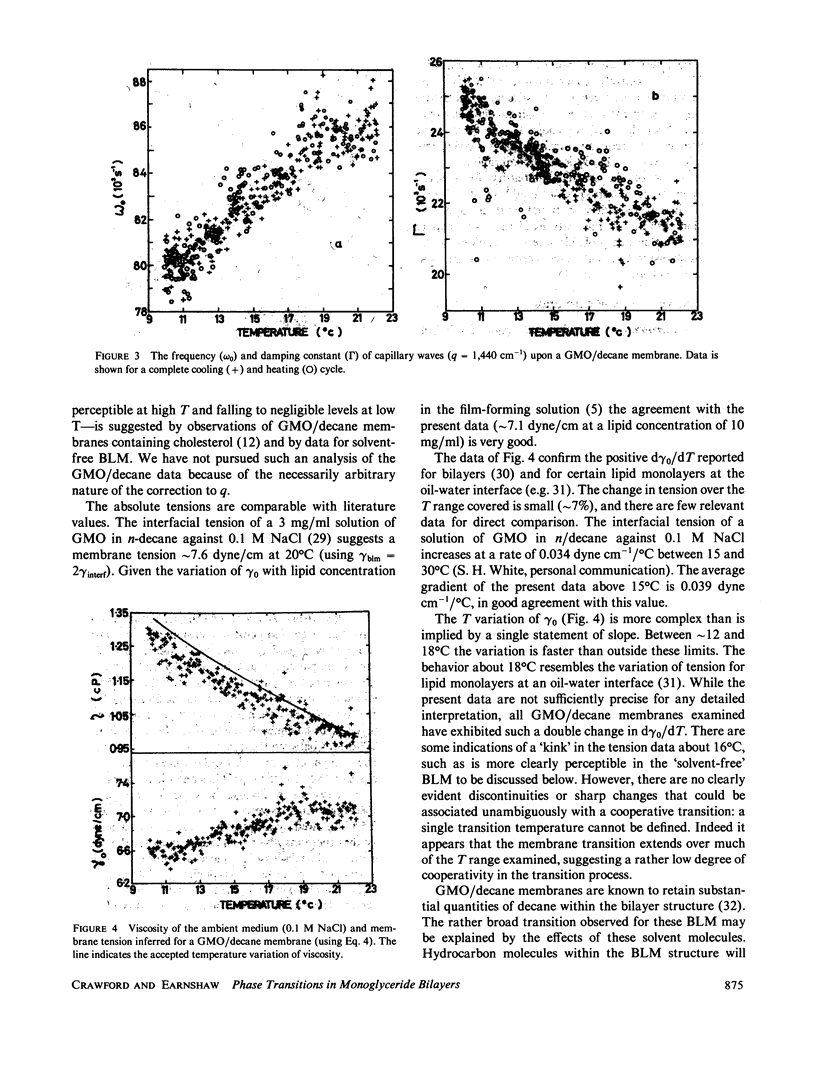
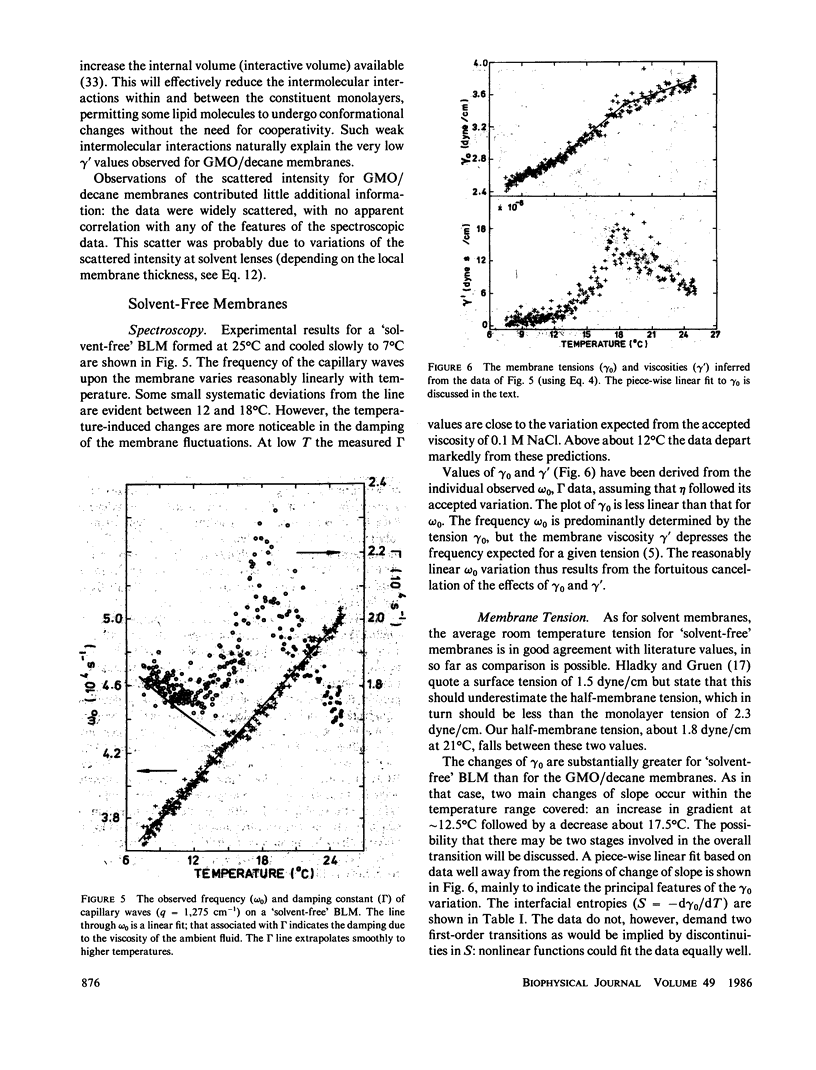
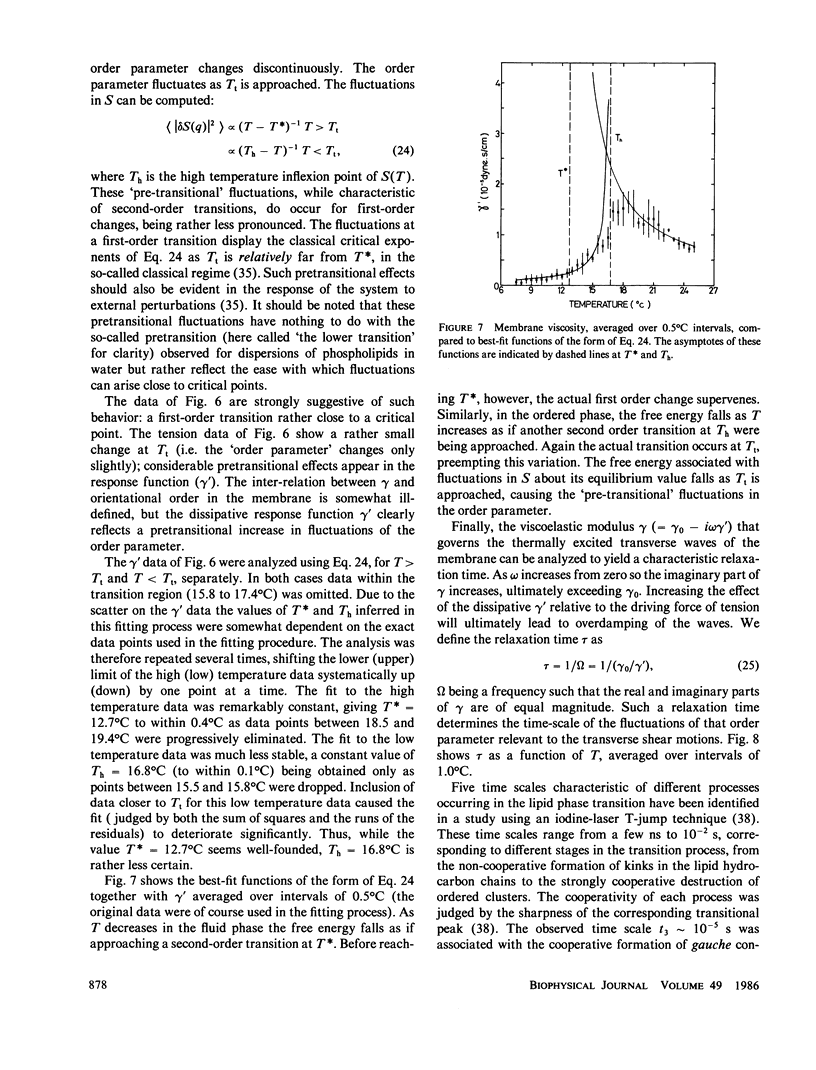
Abstract
Thermotropic phase transitions in single planar bilayers of glycerol mono-oleate have been investigated using quasi-elastic light scattering from thermally excited membrane fluctuations. In certain cases both spectroscopic and intensity information were derived from the observations. For solvent-free bilayers transitional changes were observed in several membrane parameters: in tension, viscosity and thickness, in a combination of lipid orientational order parameter and dielectric anisotropy, and in the lateral compression modulus. These changes, particularly those in membrane thickness and in the anisotropy/order combination, were clearly indicative of a chain-melting transition in the lipid molecules. The chain-melting transition temperature was identified as 16.6 +/- 0.03 degrees C (delta T 1/2 = 1.5 degrees C). The other changes tended to cluster around 12.5 and 16.6 degrees C, suggesting that a two-stage transition was involved. Analysis of pretransitional fluctuations in membrane viscosity, based on a Landau approach, suggested that at the transition the membrane was close to a critical point (T = 12.7 degrees C). Less information was accessible for membranes containing n-decane within their structure. In this case, the change in membrane tension was much smaller than in the solvent-free case and the transition was considerably broadened. These effects accord with an increase in 'interactive volume' within the bilayer due to solvent inclusion.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzi A. The application of fluorescent probes in membrane studies. Q Rev Biophys. 1975 May;8(2):237–316. doi: 10.1017/s0033583500001803. [DOI] [PubMed] [Google Scholar]
- Barton P. G., Gunstone F. D. Hydrocarbon chain packing and molecular motion in phospholipid bilayers formed from unsaturated lecithins. Synthesis and properties of sixteen positional isomers of 1,2-dioctadecenoyl-sn-glycero-3-phosphorylcholine. J Biol Chem. 1975 Jun 25;250(12):4470–4476. [PubMed] [Google Scholar]
- Cherry R. J., Chapman D. Optical properties of black lecithin films. J Mol Biol. 1969 Feb 28;40(1):19–32. doi: 10.1016/0022-2836(69)90293-9. [DOI] [PubMed] [Google Scholar]
- Crawford G. E., Earnshaw J. C. Photon correlation spectroscopy as a probe of planar lipid bilayer phase transitions. Eur Biophys J. 1984;11(1):25–33. doi: 10.1007/BF00253855. [DOI] [PubMed] [Google Scholar]
- Crilly J. F., Earnshaw J. C. Photon correlation spectroscopy of bilayer lipid membranes. Biophys J. 1983 Feb;41(2):197–210. doi: 10.1016/S0006-3495(83)84420-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilger J. P. The thickness of monoolein lipid bilayers as determined from reflectance measurements. Biochim Biophys Acta. 1981 Jul 20;645(2):357–363. doi: 10.1016/0005-2736(81)90208-x. [DOI] [PubMed] [Google Scholar]
- Evans E., Kwok R. Mechanical calorimetry of large dimyristoylphosphatidylcholine vesicles in the phase transition region. Biochemistry. 1982 Sep 28;21(20):4874–4879. doi: 10.1021/bi00263a007. [DOI] [PubMed] [Google Scholar]
- Fahey P. F., Webb W. W. Lateral diffusion in phospholipid bilayer membranes and multilamellar liquid crystals. Biochemistry. 1978 Jul 25;17(15):3046–3053. doi: 10.1021/bi00608a016. [DOI] [PubMed] [Google Scholar]
- Hladky S. B., Gruen D. W. Thickness fluctuations in black lipid membranes. Biophys J. 1982 Jun;38(3):251–258. doi: 10.1016/S0006-3495(82)84556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jähnig F. Critical effects from lipid-protein interaction in membranes. I. Theoretical description. Biophys J. 1981 Nov;36(2):329–345. doi: 10.1016/S0006-3495(81)84735-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jähnig F. Critical effects from lipid-protein interaction in membranes. II. Interpretation of experimental results. Biophys J. 1981 Nov;36(2):347–357. doi: 10.1016/S0006-3495(81)84736-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambara T., Sasaki N. A self-consistent chain model for the phase transitions in lipid bilayer membranes. Biophys J. 1984 Sep;46(3):371–382. doi: 10.1016/S0006-3495(84)84033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok R., Evans E. Thermoelasticity of large lecithin bilayer vesicles. Biophys J. 1981 Sep;35(3):637–652. doi: 10.1016/S0006-3495(81)84817-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. G. Lipid phase transitions and phase diagrams. I. Lipid phase transitions. Biochim Biophys Acta. 1977 Aug 9;472(2):237–281. doi: 10.1016/0304-4157(77)90018-1. [DOI] [PubMed] [Google Scholar]
- Lis L. J., McAlister M., Fuller N., Rand R. P., Parsegian V. A. Interactions between neutral phospholipid bilayer membranes. Biophys J. 1982 Mar;37(3):657–665. [PMC free article] [PubMed] [Google Scholar]
- Lis L. J., McAlister M., Fuller N., Rand R. P., Parsegian V. A. Measurement of the lateral compressibility of several phospholipid bilayers. Biophys J. 1982 Mar;37(3):667–672. [PMC free article] [PubMed] [Google Scholar]
- Marcelja S., Wolfe J. Properties of bilayer membranes in the phase transition or phase separation region. Biochim Biophys Acta. 1979 Oct 19;557(1):24–31. doi: 10.1016/0005-2736(79)90086-5. [DOI] [PubMed] [Google Scholar]
- Nagle J. F., Scott H. L., Jr Lateral compressibility of lipid mono- and bilayers. Theory of membrane permeability. Biochim Biophys Acta. 1978 Nov 2;513(2):236–243. doi: 10.1016/0005-2736(78)90176-1. [DOI] [PubMed] [Google Scholar]
- Pagano R. E., Cherry R. J., Chapman D. Phase transitions and heterogeneity in lipid bilayers. Science. 1973 Aug 10;181(4099):557–559. doi: 10.1126/science.181.4099.557. [DOI] [PubMed] [Google Scholar]
- Pagano R. E., Ruysschaert J. M., Miller I. R. The molecular composition of some lipid bilayer membranes in aqueous solution. J Membr Biol. 1972;10(1):11–30. doi: 10.1007/BF01867845. [DOI] [PubMed] [Google Scholar]
- Seelig A., Seelig J. The dynamic structure of fatty acyl chains in a phospholipid bilayer measured by deuterium magnetic resonance. Biochemistry. 1974 Nov 5;13(23):4839–4845. doi: 10.1021/bi00720a024. [DOI] [PubMed] [Google Scholar]
- Seelig J., Niederberger W. Two pictures of a lipid bilayer. A comparison between deuterium label and spin-label experiments. Biochemistry. 1974 Apr 9;13(8):1585–1588. doi: 10.1021/bi00705a005. [DOI] [PubMed] [Google Scholar]
- Stamatoff J., Feuer B., Guggenheim H. J., Tellez G., Yamane T. Amplitude of rippling in the P beta phase of dipalmitoylphosphatidylcholine bilayers. Biophys J. 1982 Jun;38(3):217–226. doi: 10.1016/S0006-3495(82)84551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Träuble H., Eibl H., Sawada H. Respiration--a critical phenomenon? Lipid phase transitions in the lung alveolar surfactant. Naturwissenschaften. 1974 Aug;61(8):344–354. doi: 10.1007/BF00600300. [DOI] [PubMed] [Google Scholar]
- Wallach D. F., Verma S. P., Fookson J. Application of laser Raman and infrared spectroscopy to the analysis of membrane structure. Biochim Biophys Acta. 1979 Aug 20;559(2-3):153–208. doi: 10.1016/0304-4157(79)90001-7. [DOI] [PubMed] [Google Scholar]
- White S. H. Phase transitions in planar bilayer membranes. Biophys J. 1975 Feb;15(2 Pt 1):95–117. doi: 10.1016/s0006-3495(75)85795-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S. H. Studies of the physical chemistry of planar bilayer membranes using high-precision measurements of specific capacitance. Ann N Y Acad Sci. 1977 Dec 30;303:243–265. [PubMed] [Google Scholar]


