Abstract
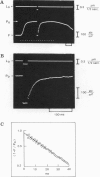
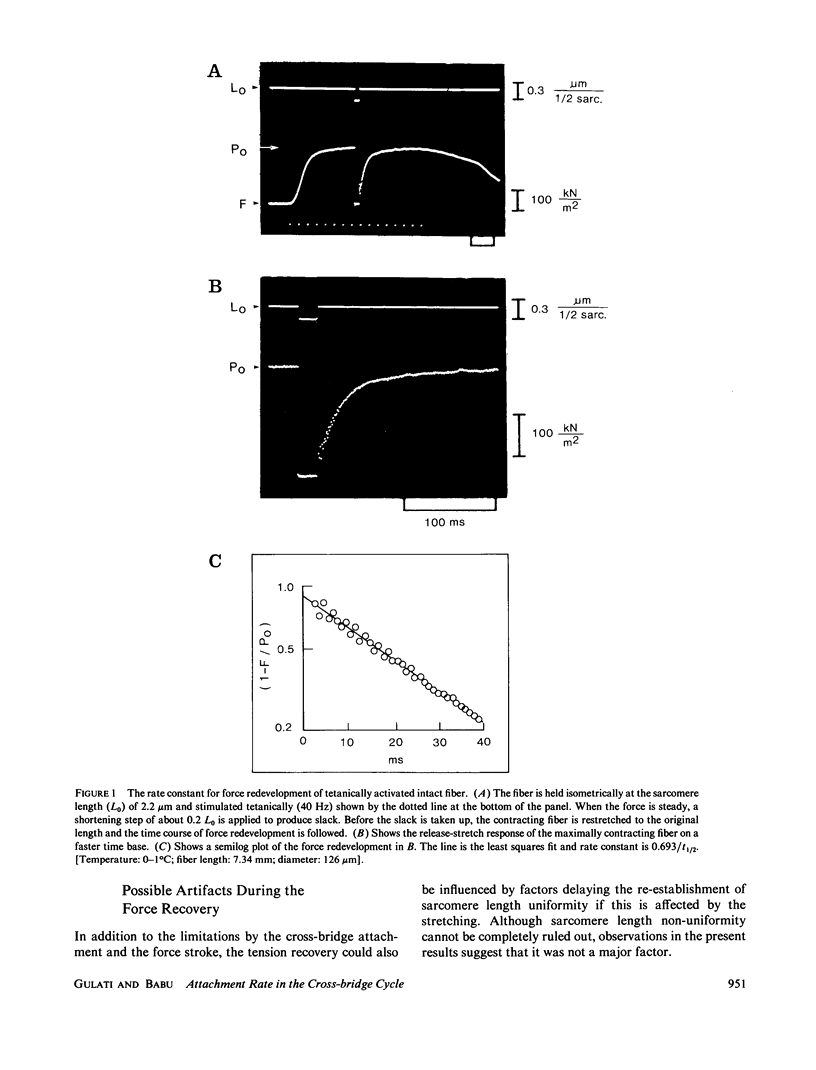
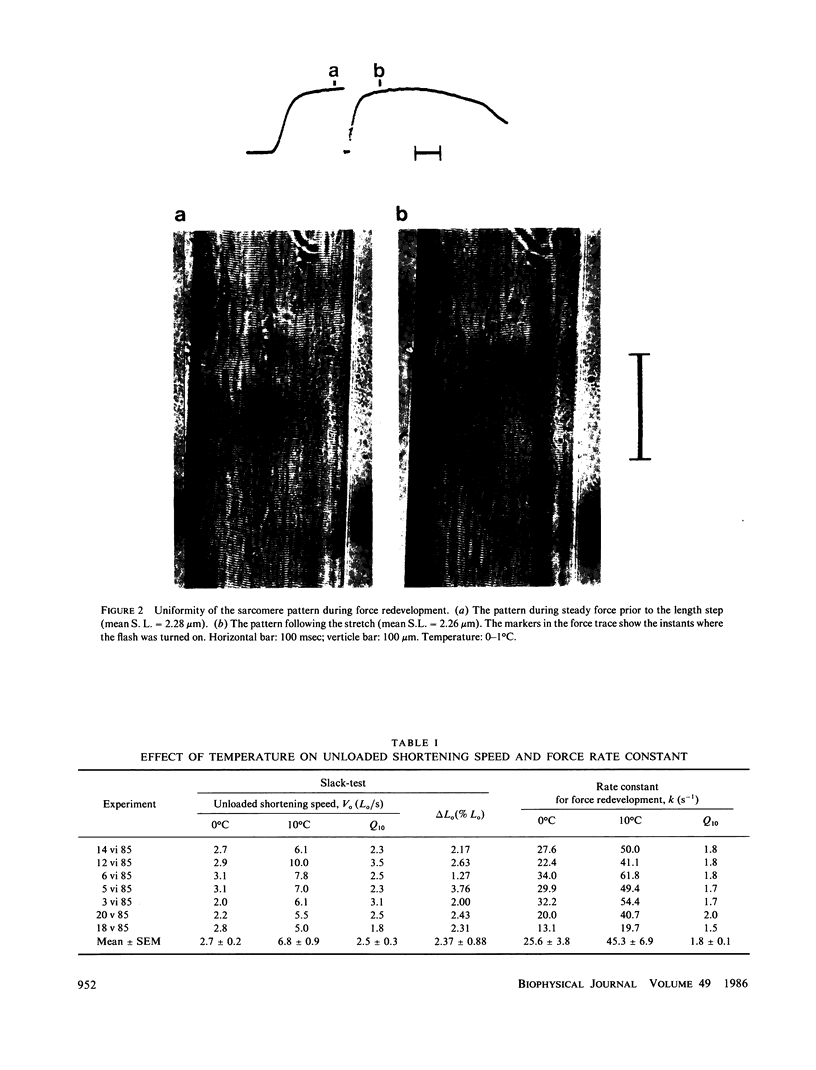
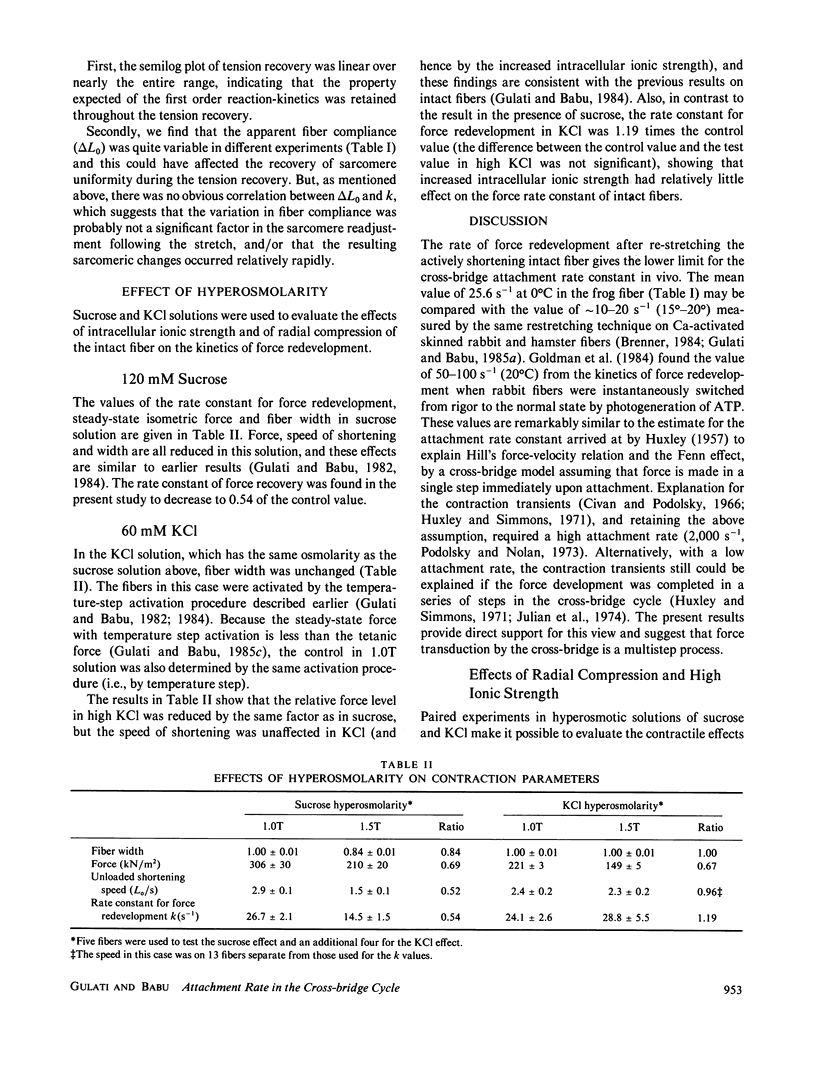
Isolated intact frog muscle fibers, while shortening with the intrinsic maximal speed, were stretched back to the original length to measure the kinetics of force redevelopment. These kinetics give information on the attachment rate constant in the cross-bridge cycle in vivo, and a value of approximately 25.6 s-1 (0 degree C) is found in the present study. We find that these kinetics were slightly less sensitive to temperature than was the unloaded shortening speed. The effect of hyperosmolarity on force redevelopment was also measured in solutions with added sucrose or KCl. The rate constant was nearly halved with 120 mM sucrose, but there was practically no effect with isosmotic (60 mM) KCl. These results indicate that the rate constant of force redevelopment is insensitive to raised intracellular ionic strength. In sucrose, the fiber width was also compressed, and the attenuation of the rate constant of force redevelopment in this case is consequently attributed to the decrease in interfilament space. The order of magnitude of the rate constant found in this study suggests that tension transduction by a cross-bridge, during each turnover cycle, requires a series of elementary steps following the attachment.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyle P. J., Conway E. J. Potassium accumulation in muscle and associated changes. J Physiol. 1941 Aug 11;100(1):1–63. doi: 10.1113/jphysiol.1941.sp003922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civan M. M., Podolsky R. J. Contraction kinetics of striated muscle fibres following quick changes in load. J Physiol. 1966 Jun;184(3):511–534. doi: 10.1113/jphysiol.1966.sp007929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A., Hwang J. C. The force-velocity relationship in vertebrate muscle fibres at varied tonicity of the extracellular medium. J Physiol. 1977 Jul;269(2):255–272. doi: 10.1113/jphysiol.1977.sp011901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczi M. A., Homsher E., Trentham D. R. The kinetics of magnesium adenosine triphosphate cleavage in skinned muscle fibres of the rabbit. J Physiol. 1984 Jul;352:575–599. doi: 10.1113/jphysiol.1984.sp015311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman Y. E., Hibberd M. G., Trentham D. R. Initiation of active contraction by photogeneration of adenosine-5'-triphosphate in rabbit psoas muscle fibres. J Physiol. 1984 Sep;354:605–624. doi: 10.1113/jphysiol.1984.sp015395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati J., Babu A. Contraction kinetics of intact and skinned frog muscle fibers and degree of activation. Effects of intracellular Ca2+ on unloaded shortening. J Gen Physiol. 1985 Oct;86(4):479–500. doi: 10.1085/jgp.86.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati J., Babu A. Critical dependence of calcium-activated force on width in highly compressed skinned fibers of the frog. Biophys J. 1985 Nov;48(5):781–787. doi: 10.1016/S0006-3495(85)83836-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati J., Babu A. Intrinsic shortening speed of temperature-jump-activated intact muscle fibers. Effects of varying osmotic pressure with sucrose and KCl. Biophys J. 1984 Feb;45(2):431–445. doi: 10.1016/S0006-3495(84)84166-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati J., Babu A. Tonicity effects on intact single muscle fibers: relation between force and cell volume. Science. 1982 Feb 26;215(4536):1109–1112. doi: 10.1126/science.6977845. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The effect of sudden changes in ionic concentrations on the membrane potential of single muscle fibres. J Physiol. 1960 Sep;153:370–385. doi: 10.1113/jphysiol.1960.sp006540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Hibberd M. G., Dantzig J. A., Trentham D. R., Goldman Y. E. Phosphate release and force generation in skeletal muscle fibers. Science. 1985 Jun 14;228(4705):1317–1319. doi: 10.1126/science.3159090. [DOI] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley A. F. The activation of striated muscle and its mechanical response. Proc R Soc Lond B Biol Sci. 1971 Jun 15;178(1050):1–27. doi: 10.1098/rspb.1971.0049. [DOI] [PubMed] [Google Scholar]
- Inoue A., Takenaka H., Arata T., Tonomura Y. Functional implications of the two-headed structure of myosin. Adv Biophys. 1979;13:1–194. [PubMed] [Google Scholar]
- Julian F. J., Sollins K. R., Sollins M. R. A model for the transient and steady-state mechanical behavior of contracting muscle. Biophys J. 1974 Jul;14(7):546–562. doi: 10.1016/S0006-3495(74)85934-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B. The relation between force and speed in muscular contraction. J Physiol. 1939 Jun 14;96(1):45–64. doi: 10.1113/jphysiol.1939.sp003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymn R. W., Taylor E. W. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry. 1971 Dec 7;10(25):4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- Palmer L. G., Gulati J. Potassium accumulation in muscle: a test of the binding hypothesis. Science. 1976 Oct 29;194(4264):521–523. doi: 10.1126/science.1085986. [DOI] [PubMed] [Google Scholar]
- Siemankowski R. F., Wiseman M. O., White H. D. ADP dissociation from actomyosin subfragment 1 is sufficiently slow to limit the unloaded shortening velocity in vertebrate muscle. Proc Natl Acad Sci U S A. 1985 Feb;82(3):658–662. doi: 10.1073/pnas.82.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein L. A., Greene L. E., Chock P. B., Eisenberg E. Rate-limiting step in the actomyosin adenosinetriphosphatase cycle: studies with myosin subfragment 1 cross-linked to actin. Biochemistry. 1985 Mar 12;24(6):1357–1363. doi: 10.1021/bi00327a013. [DOI] [PubMed] [Google Scholar]
- Trentham D. R. The twelfth Colworth Medal lecture. The adenosine triphosphatase reactions of myosin and actomyosin and their relation to energy transduction in muscle. Biochem Soc Trans. 1977;5(1):5–22. doi: 10.1042/bst0050005. [DOI] [PubMed] [Google Scholar]