Abstract
Recently, a role for B cells in the pathogenesis associated with infection by Leishmania (Leishmania mexicana complex and L. donovani) has been established. In the case of L. mexicana complex parasites (L. mexicana, L. pifanoi, and L. amazonensis), a critical role for immunoglobulin G-mediated mechanisms for the amastigote stage in the host is evident; however, the immunological mechanisms involved remain to be established. In vitro analysis of the kinetics of parasite uptake by macrophages failed to indicate a major effect of antibody opsonization. Given the importance of CD4+ T cells in the development of disease caused by these parasites, the possibility that the lack of pathogenesis was due to the lack of development of an immune response at the local site (draining lymph node and/or cutaneous site) was explored. Interestingly, the level of CD4+-T-cell activation (proliferation and cytokine) in draining lymph nodes from mice lacking circulating antibody (resistant) was found to be comparable to that in nodes from wild-type mice (susceptible) at 2, 5, and 10 weeks postinfection. However, antibody-deficient animals had markedly reduced numbers of monocytes and lymphocytes recruited or retained at the site of cutaneous infection in comparison to wild-type mice, indicating a selective impairment in the local cutaneous immune response. In vitro antigen presentation studies employing tissue-derived (opsonized) amastigotes demonstrated that L. pifanoi-infected FcR−/− macrophages, in contrast to comparably infected wild-type cells, failed to activate Leishmania antigen-specific T lymphocytes. These data, taken together, suggest that one possible mechanism for the role of antibody in pathogenesis may be to mediate parasite uptake and regulate the immune response at the local cutaneous site of infection.
Infection with Leishmania parasites can cause a spectrum of diseases, ranging from cutaneous, diffuse cutaneous and mucocutaneous lesions to visceral involvement. Cutaneous leishmaniasis is caused by members of the Leishmania major complex in the Old World and by members of the L. mexicana and L. braziliensis complexes in the New World. In cutaneous leishmaniasis, macrophages carry the major parasite load and are the most important effector cells for the destruction of intracellular Leishmania parasites via production of nitric oxide. Macrophage-mediated killing is up-regulated through cytokines (gamma interferon [IFN-γ] and heat-labile enterotoxin/tumor necrosis factor) produced by activated T cells (1, 37, 41); conversely, the production of cytokines such as interleukin 10 (IL-10) and transforming growth factor β can prevent macrophage activation and parasite killing (3-5, 14, 18).
The dimorphic Leishmania parasites exist as extracellular flagellated promastigotes in the insect vector and as obligatory intracellular amastigotes in the mammalian host. The internalization of the promastigote form has been shown to be mediated by the mannose-fucose receptor (42, 43), the fibronectin receptor (30, 33), and the complement receptors CR1 and CR3 (11, 24, 25) on the surface of host macrophages. Since promastigotes do not persist within the mammalian host, amastigotes are responsible for maintaining and spreading infection within the host. Amastigotes accomplish this by adhering to and infecting adjacent macrophages, but less is known of the molecules that mediate entry of the amastigote form into host cells. Tissue-derived amastigotes, in contrast to promastigotes, are known to be opsonized with antibody; studies indicate that antibody, in part, mediates the internalization of these amastigotes via Fc receptors (FcR) into host cells (12, 21, 26). Further, it has been shown previously that the maintenance of infection with L. mexicana complex parasites (L. amazonensis, L. mexicana, and L. pifanoi) is impaired in the absence of circulating antibody, thus establishing a critical role for antibody in the pathogenesis associated with infection by members of the L. mexicana complex (15, 26).
In the present study, in vitro experiments investigated the effect of antibody on the kinetics of amastigote uptake as well as the effect of antibody upon antigen presentation by amastigote-infected macrophages. Further, the role of antibody in vivo in the ongoing immune responses at the cutaneous site of infection and/or within the draining lymph node was examined. To analyze the mechanism(s) underlying the role of antibody and/or B cells in vivo, JHD mice that lack B cells (10) and JHD mice reconstituted by transgenesis (which contain otherwise functional B cells that do not secrete immunoglobulin [Ig] [mIgM/JHD]) (13) were employed. The results from these studies extend previous observations that IgG is essential for the pathogenesis caused by L. pifanoi infection and establish that the effect of antibody on the host immune response is selectively expressed at the cutaneous site of infection.
MATERIALS AND METHODS
Parasites.
L. pifanoi (MHOM/VE/60/Ltrod) amastigotes were maintained at 31°C in F-29 medium containing 20% heat-inactivated fetal bovine serum (HIFBS; GIBCO BRL, Grand Island, N.Y.) as previously reported (36). L. major (WR309; MHOM/IS/79/LRCL251) promastigotes were grown at 23°C in complete Schneider's Drosophila medium supplemented with 20% HIFBS and 10 μg of gentamicin/ml. Tissue-derived L. major amastigotes were obtained from 2-month-infected wild-type mice as previously described (20).
Mice.
Wild-type BALB/c mice were obtained from the National Cancer Institute (Frederick, Md.), and the Fc receptor gamma chain (FcγR) knockout mice (BALB/cByJMTac-Fcer1gtm1), deficient in FcγRI, FcγRIII, and FcɛRI, were purchased from Taconic (Germantown, N.Y.). The JH knockout strain (JHD) and the transgenic mIgM/JHD strain with functional B cells but no circulating Ig have been described previously (10, 13).
Infection of mice.
Six to eight mice per group were infected in their hind feet with 2 × 106 cultured L. pifanoi amastigotes or tissue-derived L. major amastigotes that had been incubated overnight in low-pH medium to reduce the level of opsonizing antibodies (16), as described previously. The course of infection was monitored by measurement of lesion size using a dial gauge caliper. At designated periods, mice were sacrificed to determine parasite burdens at the site of infection by limiting dilution analysis, as previously described (36). For analysis of immunological parameters and events at the lesion site, mice were infected intradermally in the ears with 2 × 106 cultured L. pifanoi amastigotes.
In vitro macrophage infection.
The J774A.1 macrophage cell line was obtained from the American Type Culture Collection (Rockville, Md.) and was maintained in RPMI medium supplemented with 10% HIFBS and 10 μg of gentamicin/ml. For studies of amastigote internalization, resident peritoneal macrophages, from wild-type BALB/c or FcγR−/− mice, were washed from the peritoneal cavity of mice with cold RPMI medium. Macrophages were incubated overnight on glass coverslips, at 105 cells/coverslip, in RPMI containing 10% HIFBS, and nonadherent cells were removed by extensive washing with warm culture medium prior to use. Cells were incubated with parasites at a ratio of 1 parasite per macrophage. Parasites used for infection were prepared as follows: L. pifanoi axenic amastigotes were obtained from culture, and opsonized L. pifanoi amastigotes were prepared by incubating 106 axenic amastigotes on ice for 30 min with serum isolated from BALB/c mice chronically infected with L. pifanoi. Nonbound antibodies were removed by washing. Tissue-derived amastigotes were obtained from the lesions of infected BALB/c mice as described previously (16). At different time points after incubation with parasites, coverslips were washed and immediately fixed with 2% paraformaldehyde. Parasites inside macrophages were detected by staining with 4′,6′-diamidino-2-phenylindole (DAPI) and visualization with a Leitz fluorescence microscope, model Orthoplan 2 (Wetzlar, Germany). Results were expressed as a percentage of infected macrophages per 200 phagocytic cells.
T-cell proliferation assays.
At 2, 5, and 10 weeks postinfection the draining lymph nodes from the ears of infected animals were excised and cell suspensions were prepared by mincing between frosted glass slides. The CD4+ T cells were then isolated by negative selection using monoclonal antibodies (MAb) to CD8, major histocompatibility complex (MHC) class II (MHC-II), B220, and FcR, followed by incubation with anti-mouse and anti-rat Ig-coated magnetic beads (PerSeptive Biosystems, Framingham, Mass.). T-cell-depleted splenocytes obtained from uninfected BALB/c mice were used as a source of antigen-presenting cells (APCs). These were prepared using MAb to Thy-1, CD8, and CD4, followed by complement-mediated lysis and treatment with 50 μg of mitomycin C/ml. CD4+ T cells from the various infected animal groups were cultured in 96-well flat-bottom plates at 2 × 105 cells per well together with 2 × 105 APCs in the presence of various concentrations of parasite (L. pifanoi amastigote) lysate. All assays were set up in Click's medium (Irvine Scientific, Santa Ana, Calif.) containing 5% fetal bovine serum. After 5 days of culture, supernatants were collected for cytokine analysis and the cultures were pulsed overnight with 1 μCi of [3H]thymidine/well to measure proliferation.
For in vitro infection experiments, peritoneal exudate cells (PECs) were recovered from mice injected 4 days prior with 1.5 ml of thioglycolate. These cells were plated at 5 × 104 per well and then infected after overnight culture with tissue-derived amastigotes at 5:1 ratio of parasites to macrophages. After 16 to 24 h of culture at 34°C, the infected cells were processed for T-cell recall experiments as described previously (16). The level of infection in PECs from FcγR−/− mice and wild-type mice at 16 h postincubation was assessed by fluorescence microscopy examination with DAPI staining. CD4+ T cells from lymph nodes of mice immunized 9 days earlier in the hind foot with frozen and thawed Leishmania antigen were added to macrophages, and T-cell proliferation assays were carried out as described above.
Cytokine measurement.
Culture supernatants were measured for the presence of IFN-γ and IL-4 using enzyme-linked immunosorbent assay kits purchased from Endogen (Woburn, Mass.) and IL-10 using a kit from BD Pharmingen (San Diego, Calif.).
Histological analysis.
Infected ears were fixed in 10% phosphate-buffered formalin and embedded in paraffin, and transversal sections were stained using hematoxylin and eosin at the Yale University School of Medicine Dermatopathology Facility.
Preparation of cell suspensions from mouse ear.
Cells from the infected ears were recovered as previously described (6), with some modifications. Briefly, at 2, 5, and 10 weeks after intradermal inoculation, the ears were collected. The ventral and dorsal dermal sheets were separated and transferred, dermal side down, on culture medium into a 24-well plate for 2 to 3 h. Each well contained 1 ml of RPMI 1640 supplemented with 10% HIFBS, 1 mg of collagenase (Boehringer)/ml, and 10 μg of brefeldin A (GolgiPlug; Pharmingen)/ml. The cell populations spontaneously emigrating from the dermis were recovered, filtered through nylon mesh, and counted.
Multiparameter analysis by flow cytometry.
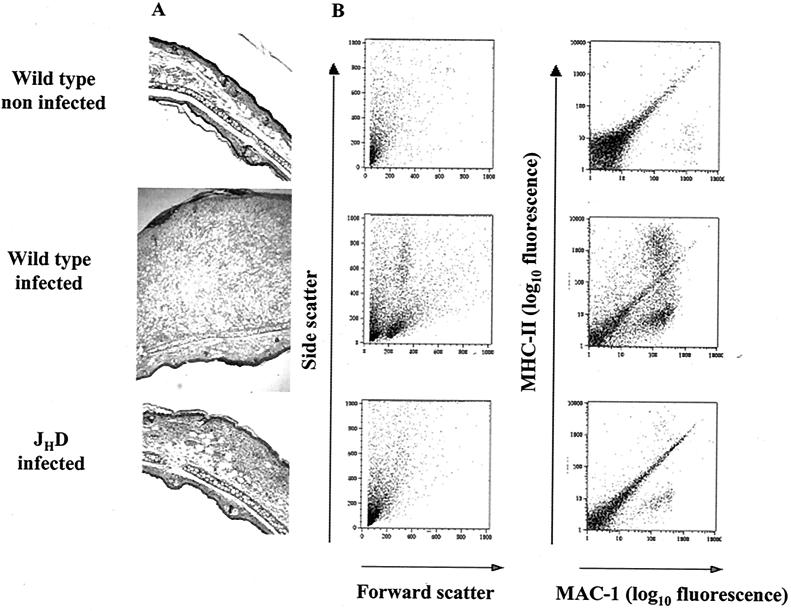
Cells emigrating from the ear dermis were washed with phosphate-buffered saline containing 1% bovine serum albumin and 0.06% NaN3 (staining medium) and were incubated with MAb anti-FcγIIIR and -IIR (FcBlock, Pharmingen). The double or triple staining was performed using directly conjugated antibodies (anti-CD4-fluorescein isothiocyanate, clone RM4-5; anti-CD8a-CyChrome, clone 53-6.7; anti-MAC-1-fluorescein isothiocyanate, clone M1/70.15; and anti-MHC-II-phycoerythrin, clone 2G9), and each incubation was conducted for 30 min on ice. The dermal cells were identified by characteristic size (forward scatter) and granulosity (side scatter) combined with two-color cell surface marker analysis. The lymphocytes were identified by their smallness, along with CD4 or CD8 expression. Monocytes and activated macrophages or dendritic cells (APCs) were identified as MAC-1 positive and MHC-II low or negative and MAC-1 positive (CD116) and MHC-II positive (7).
RESULTS
Kinetics of macrophage infection by L. pifanoi amastigotes.
It has been shown previously that there are differences in the course of L. pifanoi or L. amazonensis disease in wild-type mice from that in antibody-deficient mice (14; see also Fig. 6). One possible explanation for the observed differences in disease progression between wild-type and antibody-deficient mice is that parasite internalization by macrophages is reduced, due to the absence of parasite opsonization and consequent internalization via Fc receptors.
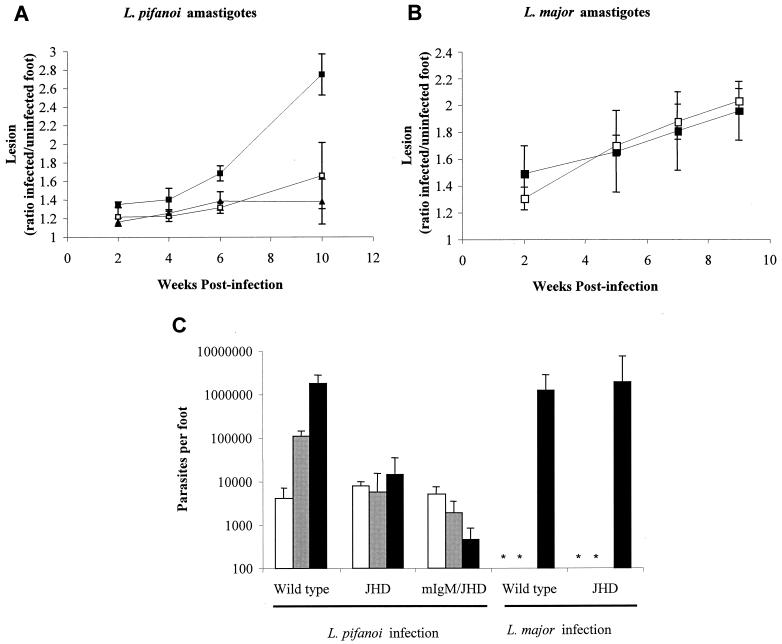
FIG. 6.
Course of infection by either L. pifanoi (A) or L. major (B) amastigotes in B-cell-deficient mice. The course of infection in JHD (□), mIgM/JHD (▴), or wild-type BALB/c (▪) mice was monitored after infection by measuring lesion development. Each data point is the mean from six to eight mice, and the bars represent standard errors of the means. (C) Parasite burdens from footpads of infected mice. At 2 (□), 5 (▪), and 10 (▪) weeks postinfection, infected feet from mice inoculated with L. pifanoi or L. major amastigotes were quantitatively assessed for parasites by a limiting dilution assay. Each data point is the mean from three mice, and the bars represent standard errors of the means. *, not determined. These results are representative of two separate experiments.
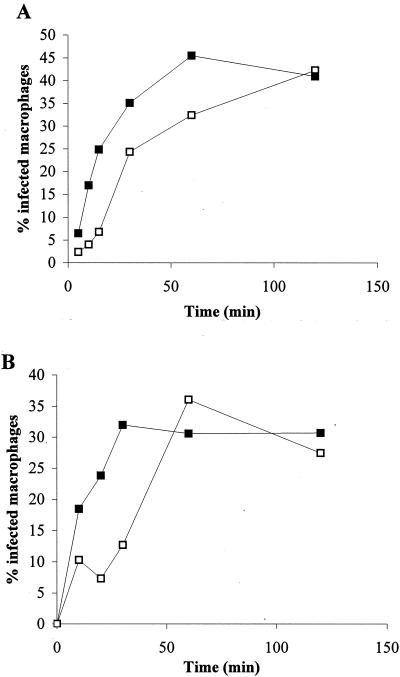
The in vitro kinetics of parasite internalization was analyzed in two situations: (i) in the presence and/or absence of opsonizing antibodies and (ii) in the presence and/or absence of Fc receptors. Shown in Fig. 1A are the kinetics of parasite internalization by J774A.1 macrophages using either axenic amastigotes or amastigotes opsonized with antibody. Although initially the rate of uptake of antibody-opsonized amastigotes is more rapid, the level of infection found for the nonopsonized organisms is only slightly retarded and, by 2 h, identical numbers of infected macrophages are detected in both groups. Similar results were found for experiments employing peritoneal exudate macrophages (data not shown). In addition tissue-derived amastigotes (which are naturally opsonized) were able to infect macrophages from FcγR−/− mice (Fig. 1B). In this last case, although the initial kinetics of uptake were slower, the percentage of infected macrophages after 1 h of incubation was identical for both types of macrophages (wild type or FcγR−/−).
FIG. 1.
Kinetics of macrophage infection with L. pifanoi. (A) J774A.1 macrophages were incubated with L. pifanoi axenic amastigote (□) or amastigotes opsonized with antibodies (▪). (B) Peritoneal resident macrophages from wild-type BALB/c (▪) or BALB/c FcγR−/− mice (□) were incubated with tissue-derived amastigote. Infections were carried out at a ratio of 1:1 of parasites to macrophages. At indicated time points, cells were fixed and stained with DAPI, and the percentage of infected macrophages was determined microscopically from the analysis of 200 phagocytic cells. These results are representative of three independent determinations.
Taken together, these data suggest that amastigote uptake by macrophages is not solely dependent on antibody-mediated opsonization. This possibility is in agreement with the results obtained by Guy and Belosevic (12), where they observed that additional receptors to FcR and CR3 may also participate in the uptake of tissue-derived amastigotes.
Analysis of immunological parameters of infected mice.
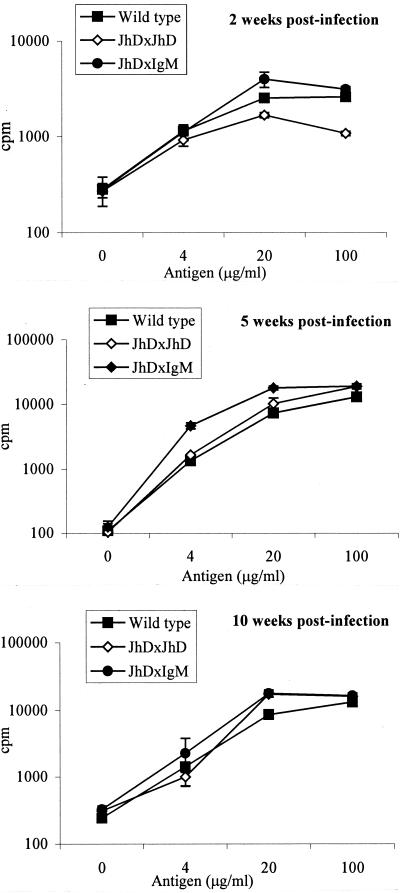
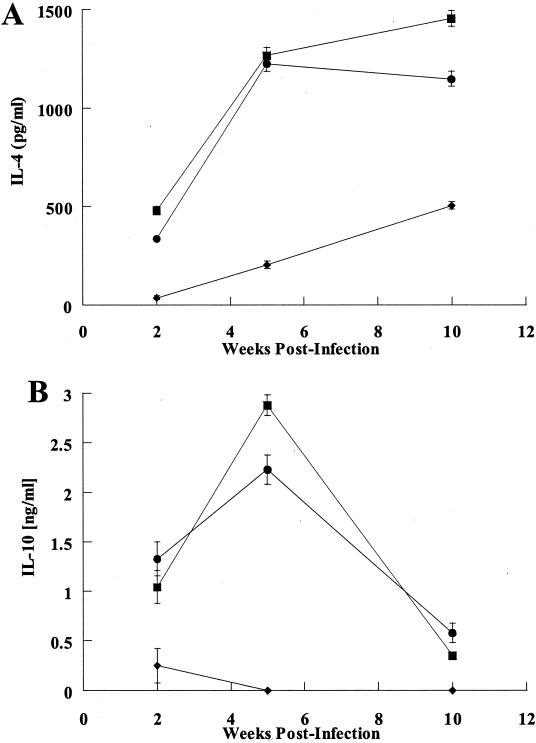
As amastigote uptake by macrophages did not appear to be limited or regulated by antibody-mediated mechanisms, the possibility that the immune responses to infection might differ was next examined in wild-type and B-cell-mutant mice. For this purpose, CD4+-T-cell sensitization in the draining lymph nodes was assessed at various times after infection. Figure 2 shows in vitro recall responses to parasite antigen of CD4+ T cells derived from draining lymph nodes after 2, 5, or 10 weeks (Fig. 2) of infection with L. pifanoi amastigotes. The magnitude of proliferation of cells from wild-type, JHD, and mIgM/JHD mouse groups was similar at all time points analyzed. When supernatants from the proliferation assays were analyzed for the presence of cytokines, similar amounts of IL-4 and IL-10 were detected from the CD4+ T cells of wild-type and mIgM/JHD mice (Fig. 3). However, the CD4+ T cells from JHD mice did not secrete IL-10 and produced low levels of IL-4. As mIgM/JHD mice (like JHD mice) are unable to sustain the L. pifanoi amastigote infection (15) (also see Fig. 6), these results indicate that IL-4 and IL-10 production by lymph node CD4+ T cells is not sufficient to maintain the pathogenesis. The lower level of cytokine production in JHD mice than in mIgM/JHD mice seems to indicate that the low secretion of IL-4 and IL-10 is related to a specific lack of functional B cells and not to the inability to sustain the infection. It should be noted that, in all cases, the levels of secreted IFN-γ were below detection (data not shown).
FIG. 2.
Recall responses of CD4+ T cells obtained from lymph nodes of L. pifanoi amastigote-infected mice. CD4+ T cells were recovered from infected wild-type BALB/c, JHD, or mIgM/JHD mice at 2, 5, and 10 weeks postinfection. The cells were then stimulated in an in vitro proliferation experiment as described in Materials and Methods using T-cell-depleted splenocytes as APCs. Bars represent standard deviation. These results are representative of two separate experiments.
FIG. 3.
Cytokine production by proliferating CD4+ T cells. CD4+ T cells were recovered from infected wild-type BALB/c (▪), JHD (♦), or mIgM/JHD (•) mice at different times postinfection. The cells were then stimulated in an in vitro proliferation experiment (100 μg of antigen) as described in Materials and Methods, and the supernatants were analyzed for IL-4 (A) or IL-10 (B) production by enzyme-linked immunosorbent assay. It should be noted that, in all cases, the levels of secreted IFN-γ were below detection (data not shown). These results are representative of two separate determinations. Bars represent standard deviation.
Thus, a deficiency in CD4+-T-cell activation within secondary lymphoid organs does not account for the lack of pathogenesis observed in the genetically deficient mice.
Histological analysis and analysis of immunological events at the lesion site.
A potentially more important site of antigen presentation is the cutaneous site of infection. In order to analyze the immunological responses at the lesion site, wild-type, JHD, and mIgM/JHD mice were infected with 2 × 106 L. pifanoi axenic amastigotes intradermally in the ear pinnae. The evolution of the ear lesions was comparable to that obtained when mice were infected in the foot as evaluated by measuring lesion size and parasite burdens (15; data not shown). At 2, 5, and 10 weeks postinfection, mice were sacrificed and cells present in the inflammatory dermal site were recovered and prepared for histological and/or flow cytometry analysis employing cell surface determinants for T cells, APCs (dendritic cells and activated macrophages [as described in Materials and Methods]). While in wild-type mice the number of cells recovered increased significantly with time postinfection (Table 1), mice lacking functional B cells showed little or no inflammation and no appreciable increase in total cells at the lesion site, correlating with the magnitude of inflammation observed (Fig. 4A). In wild-type mice, the ongoing cutaneous response to infection led to increasing numbers of lymphocytes, mainly CD4+ T cells. Recruitment of phagocytes into the lesion site in wild-type mice was also found; increases in the numbers of monocytes (Table 1) and APCs (dendritic cells or activated macrophages) (Fig. 4) were observed. In contrast in B-cell mutant mice, recruitment of lymphocytic or inflammatory cells was dramatically reduced (Fig. 4B and Table 1). It should be noted that, at 2 weeks postinfection, all three groups of mice (wild type, JHD, and mIgM/JHD) had comparable parasite burdens (two independent experiments; data not shown); hence, parasites were not apparently cleared through a heightened innate immune response in the B-cell-deficient mice. Subsequently, parasite burdens in the B-cell-deficient mice decreased, while the burdens in wild-type mice increased (15); the later development of differences in parasite burdens is consistent with the development of an IgG response and the requirement of antibody for chronic persistent parasitemia.
TABLE 1.
Leukocytes present in the dermal compartment after intradermal inoculation of L. pifanoi amastigote into the earsa
| Mouse type | No. of wk postinfection | Total no. of cells (103) | No. of lymphocytes
|
No. of monocytes (103) | |
|---|---|---|---|---|---|
| CD4+ (102) | CD8+ (102) | ||||
| Wild type | 2 | 100 (±2.0) | 7.4 (±0.14) | 0.3 (±0.06) | 13 (±0.30) |
| 5 | 450 (±9.0) | 75 (±1.5) | 4.5 (±0.09) | 61 (±1.2) | |
| 10 | 1,300 (±26.0) | 170 (±3.4) | 110 (±2.2) | 260 (±5.2) | |
| JHD | 2 | 100 (±2.0) | 20 (±0.4) | 2.9 (±0.06) | 7 (±0.14) |
| 5 | 120 (±2.4) | 0.7 (±0.01) | 1.2 (±0.02) | 5 (±0.1) | |
| 10 | 150 (±3.0) | 0.5 (±0.01) | 0.5 (±0.01) | 7.5 (±0.15) | |
| mIgM/JHD | 2 | 100 (±2.0) | 22 (±0.44) | 2.7 (±0.05) | 5.4 (±0.1) |
| 5 | 200 (±4.0) | 26 (±0.52) | 5.2 (±0.10) | 74 (±1.48) | |
| 10 | 220 (±4.4) | 1 (±0.02) | 0.5 (±0.01) | 31 (±0.62) | |
Mice were infected intradermally with 2 × 106 L. pifanoi axenic amastigotes, and at the indicated time postinfection, ears were recovered and processed as described in Materials and Methods. Standard deviations are indicated in parentheses. The results presented are representative of three independent experiments.
FIG. 4.
Analysis of leukocyte recruitment in the cutaneous lesion site. Mice were infected intradermally with 2 × 106 L. pifanoi axenic amastigotes, and at different times postinfection, ears were recovered. (A) Hematoxylin- and eosin-stained transverse sections; at 5 weeks postinfection, serial sections through the inoculation site were prepared, and the section showing the greatest transverse thickness was chosen for presentation. Magnification, ×100. (B) Representation of two-parameter dot plots of epidermal cells recovered from mice at 10 weeks postinfection and prepared for flow cytometry analysis as described in Materials and Methods. Cells were identified as follows: lymphocytes, medium size (forward scatter) and medium granular (side scatter); monocytes, positive for MAC-1 and negative or low for MHC-II; dendritic leukocytes and activated macrophages (APCs), positive for MAC-1 and for MHC-II. The quantification for individual cell populations (CD4+ and/or CD8+ T cells or macrophages) is shown in Table 1. These results are representative of three independent determinations.
In vitro activation of T cells by macrophages from FcγR−/− mice infected with tissue-derived amastigotes.
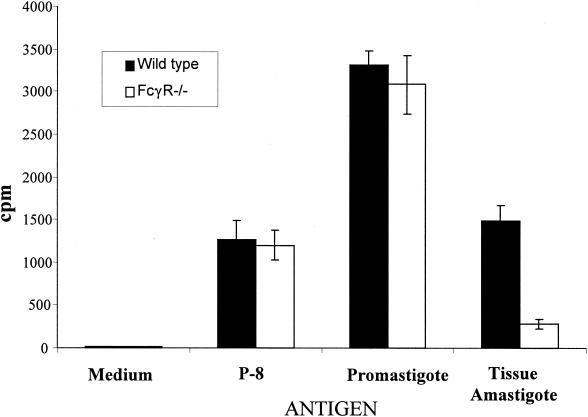
Since results suggested that cell activation at the site of infection was limited, yet no significant impairment for nonopsonized organisms in macrophage uptake was apparent, the possibility of differential antigen access or presentation by macrophages (wild type versus FcγR−/−) was assessed. Peritoneal exudate macrophages from wild-type BALB/c or FcγR−/− mice were infected with tissue-derived parasites (IgG coated) and were then assessed for their capacity to induce proliferation of CD4+ T cells previously sensitized to L. pifanoi antigens. The similarity of infection in PECs from FcγR−/− mice and wild-type mice at 16 h postincubation was confirmed by fluorescence microscopy examination using DAPI. As shown in Fig. 5, there was reduced proliferation of CD4+ T cells to PECs from FcγR−/− mice infected with tissue-derived amastigotes, compared to that to similarly infected PECs from wild-type mice. The control proliferative responses of immune CD4 T cells to macrophages (FcγR−/− or wild type) either pulsed with purified parasite antigen P-8 (fed exogenously) or infected with cultured Leishmania promastigotes were comparable. These results suggest that, when tissue-derived amastigotes are not internalized via Fc receptors, antigen presentation is significantly diminished. However, the mechanisms involved in this lack of antigen presentation (costimulatory molecule expression, cytokine and chemokine production, and/or access of Leishmania antigen to the MHC-II antigen presentation pathway) remain to be elucidated.
FIG. 5.
Antigen presentation of Leishmania antigen to CD4+ T cells by infected macrophages from BALB/c FcγR−/− or from wild-type BALB/c mice. The proliferative responses of CD4+ T cells from mice sensitized with frozen and thawed L. pifanoi amastigote antigen were employed to assess antigen presentation. PECs from FcγR−/− mice (□) or from BALB/c (▪) mice were infected with tissue-derived amastigotes at a ratio of 5:1 of parasites to macrophages, and their capacity to present endogenously synthesized Leishmania antigen to CD4+ T cells was assessed after 16 h of infection. At 16 h postinfection, the level of infected macrophages (as assessed by DAPI staining) was comparable for BALB/c-FcγR−/− and wild-type macrophages. The presentation of an affinity-purified Leishmania antigen, P-8 (1 μg/ml), and parasite antigen presentation after infection with promastigotes were used as controls in these experiments. Bars represent standard deviations. These results are representative of two independent experiments.
Taken together, these data suggest that one possible mechanism contributing to the lack of activation or recruitment at the site of infection in B-cell-deficient mice (Fig. 4) could be a reduction in antigen presentation. For L. pifanoi and L. amazonensis (in contrast to L. major), this process and the activation of CD4+ T cells are necessary for maintaining infection (35).
Course of L. pifanoi and L. major amastigote infection in mice lacking B cells.
To extend the observation that maintenance of New World Leishmania infection with L. mexicana complex parasites (L. amazonensis and L. pifanoi) is impaired in the absence of circulating antibody, the in vivo role of antibody in cutaneous leishmaniasis caused by the Old World L. major was assessed.
BALB/c wild-type and B-cell mutant mice were infected with 2 × 106 L. pifanoi axenic amastigotes or L. major tissue-derived amastigotes that had been incubated overnight in low-pH medium to reduce the level of opsonizing antibodies (16). Lesion development was monitored over 10 weeks, and mice were sacrificed at 2, 5, or 10 weeks postinfection to enumerate parasites in the lesions by limiting dilution analysis. As shown in Fig. 6A and B, in the murine model of cutaneous leishmaniasis caused by L. pifanoi, significant lesions are formed in wild-type mice, whereas in JHD mice and mIgM/JHD mice lesions are barely evident. In contrast, all groups of mice infected with L. major showed similar lesion development. The results obtained from the enumeration of parasites were consistent with the differences in lesion size (Fig. 6C). These data confirm earlier studies using L. major promastigotes (9) and indicate that the role of circulating antibody and FcγR-mediated phagocytosis appears to be different in these two models of cutaneous leishmaniasis. This difference further adds to the diversity in immunological responses detected among the related yet distinct L. major and L. mexicana (19, 35) parasites.
DISCUSSION
A critical role for B cells and antibody in the pathogenesis associated with infection caused by the amastigote stage of members of the L. mexicana complex has been established (15, 26). In the present work, we have investigated further the mechanisms leading to the fact that mice lacking B cells (JHD) or having B cells that do not secrete antibody (mIgM/JHD) do not sustain infection with L. pifanoi (L. mexicana complex) amastigotes. These experiments indicate a selective impairment of the local cutaneous response. The results of histological and flow cytometry analysis of leukocytic cells clearly show an absence of cellular recruitment (specifically activated T cells and monocytes) in B-cell-deficient mice (JHD and mIgM/JHD) at the cutaneous site of infection. This significantly contrasts to the results obtained in wild-type infected mice. Previous studies of L. amazonensis have demonstrated that CD4+-T-cell activation at the site of infection is required for the establishment of cutaneous lesion (35). One of the consequences of the lack of CD4+-T-cell activation at the lesion site is the absence of the necessary factors for the recruitment of infiltrating cells (35). Among these cells, naïve macrophages (38) are the main targets of Leishmania for maintaining the infection. Therefore, without antibody-mediated FcR uptake, local cutaneous cellular (T-cell and monocyte) activation and recruitment and retention are defective. However, it should be noted that, at the initiation of the infection, there are no Leishmania-specific circulating IgG antibodies. Consequently, these observations suggest that the pathogenesis caused by FcγR-mediated phagocytosis of L. mexicana complex parasites by macrophages is most relevant at the cutaneous site at the later stages (after initiation) of infection.
In contrast, comparable immune responses in the draining lymph node (proliferative and cytokine) were found for the B-cell-deficient and wild-type mice (2 to 10 weeks postinfection). It has been postulated that the stimulation of a primary T-cell response occurs after the transport of dendritic cell-ingested Leishmania parasites from the infected skin to the draining lymph node during the very early stages of infection (23, 27). von Stebut et al. (40) have shown that the ingestion of tissue-derived L. major amastigotes by fetal skin-derived dendritic cells leads to up-regulation of MHC-I and -II antigens, CD40, CD54, and CD86 and secretion of tumor necrosis factor alpha, IL-6, and IL-12. In contrast, Bennett et al. (8) have found that infection of bone marrow-derived dendritic cells by L. mexicana axenic amastigotes retained an immature phenotype and did not up-regulate expression of the dendritic cell activation markers. However, Qi and Soong (27) have found that tissue-isolated L. amazonensis amastigotes condition dendritic cells of a susceptible host to a state that favors activation of pathogenic IL-4-producing CD4+ T cells. Therefore, dendritic cell antigen presentation, activated as a consequence of Leishmania infection, most likely explains the presence of activated antigen-specific CD4+ T cells found in the draining lymph node of mice unable to secrete antibody (JHD and/or mIgM/JHD).
Recently it has been demonstrated that ligation of Fc receptors on inflammatory macrophages by IgG present on opsonized L. major amastigotes allows the preferential induction and production of large amounts of IL-10, which prevents macrophage activation and parasite killing (14). In addition, axenic amastigotes can readily infect macrophages (without utilizing Fc receptor-mediated mechanisms); in this case, parasite antigen presentation has been demonstrated to be minimal (16). The equilibrium between these two mechanisms of amastigote uptake, and possibly others, may allow for the survival and maintenance of the parasite without causing excessive pathology in the host. In the present study, comparable levels of IL-10 were produced by lymph node-derived CD4+ T cells from either wild-type (susceptible) or antibody-deficient (mIgM/JHD; nonsusceptible) mice. Consequently for L. pifanoi parasites, available antigen-specific T cells producing IL-10 may be necessary but not sufficient in themselves to maintain infection; the ongoing response at the cutaneous site of infection appears critical to pathogenesis. Interestingly, FcR ligation on macrophages has also been shown (2, 17, 22, 32) to result in the production of the chemokines monocyte chemoattractant protein 1 (MIP-1α) and macrophage inflammatory protein 1α (MCP-1), which promote the recruitment of monocytes. Although the roles of these chemokines in infection caused by L. major and L. donovani have been studied (28, 29, 31), the roles of MCP-1 and MIP-1α in L. mexicana complex pathogenesis and the role of parasite-specific antibody in the local cutaneous chemokine production remain to be determined. However, the role of antibody in the regulation of antigen presentation (T-cell activation) together with specific chemokine and cytokine production may interplay in the pathogenesis of L. pifanoi infection.
There is increasing evidence that the pathogenesis caused by L. pifanoi and L. amazonensis (L. mexicana complex) parasites follows a pattern different from that described for L. major cutaneous leishmaniasis. Unlike L. major-infected mice, in which a polarized differentiation of Th1 and Th2 CD4+ T cells can be detected in resistant and susceptible mice, respectively, susceptible L. amazonensis-infected mice do not uniformly show an enhanced Th2 response (1, 35). Furthermore, mice lacking functional CD4+ T cells showed no signs of lesion development up to 14 weeks postinfection with L. amazonensis (35), in contrast with the results obtained in L. major-infected mice (19). In the present study employing amastigotes, as in a previous study using promastigotes (9), evidence indicates that JHD mice infected with L. major develop lesions and have parasite burdens similar to those of wild-type mice. Here too, the role of B cells in pathogenesis seems to be different in these two models of cutaneous leishmaniasis. Further, it is of interest to note that, for L. donovani, B cells appear to regulate infection independent of IgG (34); consequently, the role of B cells in pathogenesis in Leishmania infection appears to vary dependent upon the infective species. In recent years, considerable diversity in virulence factors has been determined between L. mexicana and L. major (39). These differences in pathogenesis and virulence may be associated with the evolutionary antiquity of Leishmania, as divergences of lineages within this genus (i.e., L. major versus L. mexicana) may exceed 80 million years. The differences in immunological mechanisms that are now evident in murine models (19, 35), together with the variation in virulence factors (39), may account for the variation in the diseases caused by different Leishmania species. These different mechanisms of pathogenesis may be important to consider in the design of vaccines against leishmaniasis.
Acknowledgments
We thank M. Shlomchik for providing us with breeding pairs of JHD and mIgM/JHD mice and G. Milon for her advice in the preparation of cell suspensions from mouse tissue for analysis.
This work was supported by NIH grants D.M.-P. (AI27811) and to S.L.C. (AI39158). P.E.K. was supported by a research supplement to AI34867 under the RSUM program.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Afonso, L. C., and P. Scott. 1993. Immune responses associated with susceptibility of C57BL/10 mice to Leishmania amazonensis. Infect. Immun. 61:2952-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso, A., Y. Bayon, M. Renedo, and M. S. Crespo. 2000. Stimulation of Fc gamma R receptors induces monocyte chemoattractant protein-1 in the human monocytic cell line THP-1 by a mechanism involving I kappa B-alpha degradation and formation of p50/p65 NF-kappa B/Rel complexes. Int. Immunol. 12:547-554. [DOI] [PubMed] [Google Scholar]
- 3.Balestieri, F. M., A. R. Queiroz, C. Scavone, V. M. Costa, M. Barral-Netto, and A. Abrahamsohn Ide. 2002. Leishmania (L.) amazonensis-induced inhibition of nitric oxide synthesis in host macrophages. Microbes Infect. 4:23-29. [DOI] [PubMed] [Google Scholar]
- 4.Barral-Netto, M., A. Barral, C. E. Brownell, Y. A. Skeiky, L. R. Ellingsworth, D. R. Twardzik, and S. G. Reed. 1992. Transforming growth factor-beta in leishmanial infection: a parasite escape mechanism. Science 257:545-548. [DOI] [PubMed] [Google Scholar]
- 5.Belkaid, Y., K. F. Hoffmann, S. Mendez, S. Kamhawi, M. C. Udey, T. A. Wynn, and D. L. Sacks. 2001. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J. Exp. Med. 194:1497-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belkaid, Y., H. Jouin, and G. Milon. 1996. A method to recover, enumerate and identify lymphomyeloid cells present in an inflammatory dermal site: a study in laboratory mice. J. Immunol. Methods 199:5-25. [DOI] [PubMed] [Google Scholar]
- 7.Belkaid, Y., S. Kamhawi, G. Modi, J. Valenzuela, N. Noben-Trauth, E. Rowton, J. Ribeiro, and D. L. Sacks. 1998. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J. Exp. Med. 188:1941-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett, C. L., A. Misslitz, L. Colledge, T. Aebischer, and C. C. Blackburn. 2001. Silent infection of bone marrow-derived dendritic cells by Leishmania mexicana amastigotes. Eur. J. Immunol. 31:876-883. [DOI] [PubMed] [Google Scholar]
- 9.Brown, D. R., and S. L. Reiner. 1999. Polarized helper-T-cell responses against Leishmania major in the absence of B cells. Infect. Immun. 67:266-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, J., R. Lansford, V. Stewart, F. Young, and F. W. Alt. 1993. RAG-2-deficient blastocyst complementation: an assay of gene function in lymphocyte development. Proc. Natl. Acad. Sci. USA 90:4528-4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Da Silva, R. P., B. F. Hall, K. A. Joiner, and D. L. Sacks. 1989. CR1, the C3b receptor, mediates binding of infective Leishmania major metacyclic promastigotes to human macrophages. J. Immunol. 143:617-622. [PubMed] [Google Scholar]
- 12.Guy, R. A., and M. Belosevic. 1993. Comparison of receptors required for entry of Leishmania major amastigotes into macrophages. Infect. Immun. 61:1553-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hannum, L. G., A. M. Haberman, S. M. Anderson, and M. J. Shlomchik. 2000. Germinal center initiation, variable gene region hypermutation, and mutant B cell selection without detectable immune complexes on follicular dendritic cells. J. Exp. Med. 192:931-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kane, M. M., and D. M. Mosser. 2001. The role of IL-10 in promoting disease progression in leishmaniasis. J. Immunol. 166:1141-1147. [DOI] [PubMed] [Google Scholar]
- 15.Kima, P. E., S. L. Constant, L. Hannum, M. Colmenares, K. S. Lee, A. M. Haberman, M. J. Shlomchik, and D. McMahon-Pratt. 2000. Internalization of Leishmania mexicana complex amastigotes via the Fc receptor is required to sustain infection in murine cutaneous leishmaniasis. J. Exp. Med. 191:1063-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kima, P. E., L. Soong, C. Chicharro, N. H. Ruddle, and D. McMahon-Pratt. 1996. Leishmania-infected macrophages sequester endogenously synthesized parasite antigens from presentation to CD4+ T cells. Eur. J. Immunol. 26:3163-3169. [DOI] [PubMed] [Google Scholar]
- 17.Lendvai, N., X. W. Qu, W. Hsueh, and A. Casadevall. 2000. Mechanism for the isotype dependence of antibody-mediated toxicity in Cryptococcus neoformans-infected mice. J. Immunol. 164:4367-4374. [DOI] [PubMed] [Google Scholar]
- 18.Li, J., C. A. Hunter, and J. P. Farrell. 1999. Anti-TGF-beta treatment promotes rapid healing of Leishmania major infection in mice by enhancing in vivo nitric oxide production. J. Immunol. 162:974-979. [PubMed] [Google Scholar]
- 19.Locksley, R. M., S. L. Reiner, F. Hatam, D. R. Littman, and N. Killeen. 1993. Helper T cells without CD4: control of leishmaniasis in CD4-deficient mice. Science 261:1448-1451. [DOI] [PubMed] [Google Scholar]
- 20.Love, D. C., J. D. Esko, and D. M. Mosser. 1993. A heparin-binding activity on Leishmania amastigotes which mediates adhesion to cellular proteoglycans. J. Cell Biol. 123:759-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Love, D. C., M. Mentink Kane, and D. M. Mosser. 1998. Leishmania amazonensis: the phagocytosis of amastigotes by macrophages. Exp. Parasitol. 88:161-171. [DOI] [PubMed] [Google Scholar]
- 22.Marsh, C. B., M. D. Wewers, L. C. Tan, and B. H. Rovin. 1997. Fc(gamma) receptor cross-linking induces peripheral blood mononuclear cell monocyte chemoattractant protein-1 expression: role of lymphocyte Fc(gamma)RIII. J. Immunol. 158:1078-1084. [PubMed] [Google Scholar]
- 23.Moll, H., H. Fuchs, C. Blank, and M. Rollinghoff. 1993. Langerhans cells transport Leishmania major from the infected skin to the draining lymph node for presentation to antigen-specific T cells. Eur. J. Immunol. 23:1595-1601. [DOI] [PubMed] [Google Scholar]
- 24.Mosser, D. M., and P. J. Edelson. 1984. Activation of the alternative complement pathway by Leishmania promastigotes: parasite lysis and attachment to macrophages. J. Immunol. 132:1501-1505. [PubMed] [Google Scholar]
- 25.Mosser, D. M., T. A. Springer, and M. S. Diamond. 1992. Leishmania promastigotes require opsonic complement to bind to the human leukocyte integrin Mac-1 (CD11b/CD18). J. Cell Biol. 116:511-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peters, C., T. Aebischer, Y. D. Stierhof, M. Fuchs, and P. Overath. 1995. The role of macrophage receptors in adhesion and uptake of Leishmania mexicana amastigotes. J. Cell Sci. 108:3715-3724. [DOI] [PubMed] [Google Scholar]
- 27.Qi, H., V. Popov, and L. Soong. 2001. Leishmania amazonensis-dendritic cell interactions in vitro and the priming of parasite-specific CD4(+) T cells in vivo. J. Immunol. 167:4534-4542. [DOI] [PubMed] [Google Scholar]
- 28.Ritter, U., and H. Moll. 2000. Monocyte chemotactic protein-1 stimulates the killing of leishmania major by human monocytes, acts synergistically with IFN-gamma and is antagonized by IL-4. Eur. J. Immunol. 30:3111-3120. [DOI] [PubMed] [Google Scholar]
- 29.Ritter, U., H. Moll, T. Laskay, E. Brocker, O. Velazco, I. Becker, and R. Gillitzer. 1996. Differential expression of chemokines in patients with localized and diffuse cutaneous American leishmaniasis. J. Infect. Dis. 173:699-709. [DOI] [PubMed] [Google Scholar]
- 30.Rizvi, F. S., M. A. Ouaissi, B. Marty, F. Santoro, and A. Capron. 1988. The major surface protein of Leishmania promastigotes is a fibronectin-like molecule. Eur. J. Immunol. 18:473-476. [DOI] [PubMed] [Google Scholar]
- 31.Rousseau, D., S. Demartino, F. Anjuere, B. Ferrua, K. Fragaki, Y. Le Fichoux, and J. Kubar. 2001. Sustained parasite burden in the spleen of Leishmania infantum-infected BALB/c mice is accompanied by expression of MCP-1 transcripts and lack of protection against challenge. Eur. Cytokine Netw. 12:340-347. [PubMed] [Google Scholar]
- 32.Rovin, B. H., L. Lu, and C. B. Marsh. 2001. Lymphocytes induce monocyte chemoattractant protein-1 production by renal cells after Fc gamma receptor cross-linking: role of IL-1beta. J. Leukoc. Biol. 69:435-439. [PubMed] [Google Scholar]
- 33.Russell, D. G., and P. Talamas-Rohana. 1989. Leishmania and the macrophage: a marriage of inconvenience. Immunol. Today 10:328-333. [DOI] [PubMed] [Google Scholar]
- 34.Smelt, S. C., S. E. Cotterell, C. R. Engwerda, and P. M. Kaye. 2000. B cell-deficient mice are highly resistant to Leishmania donovani infection, but develop neutrophil-mediated tissue pathology. J. Immunol. 164:3681-3688. [DOI] [PubMed] [Google Scholar]
- 35.Soong, L., C. H. Chang, J. Sun, B. J. Longley, Jr., N. H. Ruddle, R. A. Flavell, and D. McMahon-Pratt. 1997. Role of CD4+ T cells in pathogenesis associated with Leishmania amazonensis infection. J. Immunol. 158:5374-5383. [PubMed] [Google Scholar]
- 36.Soong, L., S. M. Duboise, P. Kima, and D. McMahon-Pratt. 1995. Leishmania pifanoi amastigote antigens protect mice against cutaneous leishmaniasis. Infect. Immun. 63:3559-3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soong, L., J. C. Xu, I. S. Grewal, P. Kima, J. Sun, B. J. Longley, N. H. Ruddle, D. McMahon-Pratt, and R. A. Flavell. 1996. Disruption of CD40-CD40 ligand interactions results in an enhanced susceptibility to Leishmania amazonensis infection. Immunity 4:263-273. [DOI] [PubMed] [Google Scholar]
- 38.Sunderkotter, C., M. Kunz, K. Steinbrink, G. Meinardus-Hager, M. Goebeler, H. Bildau, and C. Sorg. 1993. Resistance of mice to experimental leishmaniasis is associated with more rapid appearance of mature macrophages in vitro and in vivo. J. Immunol. 151:4891-4901. [PubMed] [Google Scholar]
- 39.Turco, S. J., G. F. Spath, and S. M. Beverley. 2001. Is lipophosphoglycan a virulence factor? A surprising diversity between Leishmania species. Trends Parasitol. 17:223-226. [DOI] [PubMed] [Google Scholar]
- 40.von Stebut, E., Y. Belkaid, B. V. Nguyen, M. Cushing, D. L. Sacks, and M. C. Udey. 2000. Leishmania major-infected murine langerhans cell-like dendritic cells from susceptible mice release IL-12 after infection and vaccinate against experimental cutaneous leishmaniasis. Eur. J. Immunol. 30:3498-3506. [DOI] [PubMed] [Google Scholar]
- 41.Wei, X. Q., I. G. Charles, A. Smith, J. Ure, G. J. Feng, F. P. Huang, D. Xu, W. Muller, S. Moncada, and F. Y. Liew. 1995. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature 375:408-411. [DOI] [PubMed] [Google Scholar]
- 42.Wilson, M. E., and K. K. Hardin. 1988. The major concanavalin A-binding surface glycoprotein of Leishmania donovani chagasi promastigotes is involved in attachment to human macrophages. J. Immunol. 141:265-272. [PubMed] [Google Scholar]
- 43.Wilson, M. E., and R. D. Pearson. 1988. Roles of CR3 and mannose receptors in the attachment and ingestion of Leishmania donovani by human mononuclear phagocytes. Infect. Immun. 56:363-369. [DOI] [PMC free article] [PubMed] [Google Scholar]