Abstract
Interferon regulatory factor 1-deficient (IRF-1−/−) mice infected with virulent Brucella abortus 2308 at 5 × 105 CFU developed acute hepatitis similar to many natural hosts but, unlike natural hosts, IRF-1−/− mice were unable to resolve infection and died. In contrast, IRF-1−/− mice survived when infected at 5 × 105 CFU with several attenuated Brucella strains (S19, RB51, cbp, and cyd). The survival of infected IRF-1−/− mice is likely a function of the level of virulence of each Brucella strain and the extent of retained immunity. Further, these findings suggest that adaptive immunity may be important to the survival of IRF-1−/− mice since attenuated Brucella strains can protect IRF-1−/− mice against lethal challenge with virulent Brucella. Using the IRF-1−/− mouse model, the following set of criteria were identified to define Brucella virulence: (i) the day of death for 50% of mice infected with 5 × 105CFU of Brucella, (ii) the extent of liver toxicity, and (iii) the minimum immunizing dose of Brucella to protect against challenge with virulent S2308. Thus, IRF-1−/− mice are important to determining the level of Brucella virulence, to evaluating Brucella mutants for attenuation, and to investigating adaptive immunity in brucellosis.
Brucella abortus, a gram-negative facultative intracellular bacterium, is the etiologic agent of zoonotic brucellosis, whose pathologic manifestations are hepatitis, arthritis, endocarditis, and meningitis in humans and spontaneous abortion in cattle, respectively (4, 7, 17). This airborne pathogen resides and multiplies in macrophages of mammalian hosts and has been used as a model to study immune response against intracellular bacteria, as well as to investigate the mechanisms of intracellular bacterial pathogenesis (25, 26, 27). Brucella virulence is determined by infecting mice with various Brucella strains and sequentially monitoring bacterial clearance from selected organs, usually the liver and spleen (6, 9, 15a, 28).
Gamma interferon (IFN-γ) is a crucial cytokine in Brucella immunity required for macrophage bactericidal activity (2). In general, the diverse functions of IFN-γ are mediated by interferon regulatory factors (IRFs), a family of secondary transcriptional factors (18, 29). This family, which includes IRF-1 through IRF-8 and p48, is characterized by the unique “tryptophan cluster” DNA-binding region located at the N terminus. IRF-1 is strongly inducible by IFN-α/β and IFN-γ, as well as by tumor necrosis factor alpha (TNF-α), and participates in many IFN-specific signaling pathways. The immunobiology of IRF-1 is elucidated by IRF-1−/− mice. These mice are characterized by a 90% reduction of CD8+ T cells, functionally impaired natual killer (NK) cells, and dysregulation of interleukin-12 (IL-12) p40 and inducible nitric oxide synthase (iNOS) induction, which are necessary to confer proper immune protection. Thus, this mouse strain is useful for understanding the roles of IRF-1 during in vivo bacterial infections.
The clearance of Brucella from host tissues is often examined for at least 6 weeks because of the chronic nature of brucellosis, and highly virulent strains can persist for at least 20 weeks (26). Further, the chronic nature of Brucella infection in mice parallels the chronic nature of human infection (3, 20). However, determining the virulence of several Brucella strains based on the time of clearance from organs creates a formidable labor-intensive challenge. Therefore, animal models that would provide a rapid measure of Brucella virulence are needed. Previously, we reported that IRF-1−/− mice infected with 5 × 105 CFU of virulent B. abortus S2308 died from severe hepatic damage within 2 weeks postinfection, whereas RB51-infected mice survived (Ko et al., unpublished), implying a significant role for IRF-1 in Brucella immunity in vivo. However, the survival of IRF-1−/− mice infected with 5 × 105 CFU of the attenuated RB51 strain suggests that a certain level of Brucella virulence is required to induce the death of IRF-1−/− mice. IRF-1−/− mice appear to have a level of basal immunity necessary to control the infection.
No human vaccine exists against Brucella, and methods are necessary to dissect Brucella pathogenesis to reach this vaccine goal. A number of Brucella strains have been identified, but readily available criteria to rapidly distinguish and rank the level of virulence among Brucella strains are needed. Thus, the rapid death of IRF-1−/− mice infected with virulent Brucella, in contrast to the survival of IRF-1−/− mice infected with an attenuated Brucella strain, suggests that IRF-1−/− mice, as an animal model, could be useful for detecting various degrees of Brucella virulence. Here we have utilized the IRF-1−/− mouse to define the level of virulence of various Brucella strains.
The aim of the present study was to investigate both wild-type and mutant Brucella strains for virulence and thus define the criteria for Brucella virulence. We also found that immunologic memory can provide protection to IRF-1−/− mice. Here we report that the criteria of Brucella virulence among several strains is based on the following factors: (i) the rapidity of death in IRF-1−/− mice infected with Brucella at 5 × 105 CFU, (ii) the extent of hepatic damage, and (iii) the protection against Brucella virulence provided by immunizing IRF-1−/− mice with an attenuated strain that induces an adaptive immune response. The varying responses of IRF-1−/− mice against diverse Brucella strains as a function of different levels of virulence suggests the importance of this mouse, as an animal model, for dissecting bacterial pathogenesis by using attenuated, gene-disrupted bacteria. In so doing, the immunobiology of IRF-1−/− mice will be clarified, thus increasing the value of the mouse strain as an animal model.
MATERIALS AND METHODS
Bacterial strains.
B. abortus S2308, the virulent wild-type strain, and S19, a live attenuated previous U.S. vaccine strain, were kindly provided by Barbara Martin, Veterinary Services, National Animal Disease Center, Ames, Iowa. RB51, the current live attenuated U.S. vaccine strain, was a generous gift of Gerhardt G. Schurig, Virginia-Maryland Regional College of Veterinary Medicine, Virginia Polytechnic Institute and State University, Blacksburg, Va. B. abortus cyd (9) and cbp (T. A. Ficht, unpublished data) insertional mutants (kanamycin resistant [Kmr]) were provided by Thomas A. Ficht, and bap (Kmr) (15) deletion mutants were produced as previously described.
Mice.
A pair of IRF-1−/− mice, originally produced by Matsuyama et al. (19) by using C57BL/6 (H-2b) mice, was kindly donated by Tak W. Mak, Amgen Institute, Ontario Cancer Institute, University of Toronto, Toronto, Ontario, Canada. These mice strains were heterozygously bred in the Department of Animal Health and Biomedical Sciences animal care facilities, and 6- to 9-week-old mice were used for experimental infection. Prior to infection, IRF-1−/− mice were genotyped by PCR (26). Whenever possible, IRF-1+/+ mice derived from heterozygous littermates were used as controls; otherwise, C57BL/6 (H-2b) mice were used (Jackson Laboratory, Bar Harbor, Maine). Mice infected with Brucella abortus were housed in a biosafety level 3 facility in the School of Veterinary Medicine.
Infection and challenge of mice.
IRF-1+/+ and IRF-1−/− mice were injected intraperitoneally with diverse strains and CFU of B. abortus in 200 μl of phosphate-buffered saline (PBS). After 6 weeks of infection, surviving IRF-1−/− mice were challenged with 5 × 105 CFU of B. abortus S2308 in 200 μl of PBS. Infected IRF-1−/− mice were monitored daily.
Enumeration of Brucella from spleens and livers.
To count residual Brucella CFU in the spleens or livers of mice, five mice from each group were examined at each sampling period. Spleens or livers were homogenized in plastic bags with 10 ml of sterile PBS by using a Stomacher Lab Blender (Tekmar, Cincinnati, Ohio). To enumerate viable Brucella, homogenized spleen or liver samples were serially diluted 10-fold with PBS and plated on Brucella agar (Difco, Detroit, Mich.). Brucella colonies were counted after a 3-day incubation at 37°C with 5% CO2. Bacterial CFU at <100 per organ were not detected by this method.
GOT, GPT, and albumin assays.
Mice were exsanguinated from the orbital sinus and sera were collected by using Microtainer (Becton Dickinson, Rutherford, N.J.). Glutamic oxalacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT) activities, as well as the albumin concentration, were quantitatively measured by colorimetric determination with diagnostic transaminase reagents and albumin reagent (BCG), respectively, according to the manufacturer's direction (Sigma, St. Louis, Mo.). The activities of GOT and GPT were measured with 1:5 diluted sera with PBS.
Histology.
Brucella-infected mice were killed 12 days postinfection. Organs were immersed in 10% formalin, embedded, microsectioned, mounted on microscopic slides, and stained with hematoxylin and eosin. The hematoxylin and eosin-stained slides were microscopically observed.
Statistical analysis.
Data from infected mice were analyzed independently and compared by using analysis of variance and the generalized Wilcoxon rank sum test (Lifetest) in the SAS program (SAS Institute, Inc., Cary, N.C.). Infection of IRF-1−/− mice was performed twice independently, and then the results were pooled to perform the data analysis. P values of <0.05 were considered significant.
RESULTS
Survival of IRF-1−/− mice infected with different CFU counts of B. abortus S2308, S19, and RB51.
Previously, we reported that IRF-1−/− mice respond to Brucella infection in a death or survival manner, depending on the levels of virulence among injected strains at 5 × 105 CFU (15a). This result suggested that a certain level of virulence is required to induce death in IRF-1−/− mice and/or the basal immunity of IRF-1−/− mice can control the infection caused by an attenuated strain. Thus, to establish criteria to determine virulence, the response of IRF-1−/− mice infected with selected doses of B. abortus strains possessing different levels of virulence was investigated, and the results are summarized in Table 1. The response of IRF-1−/− mice was tested with three different B. abortus strains; wild-type strain S2308, the previous vaccine strain S19, and the current vaccine strain RB51. The differences in the virulence of these three strains have been well elucidated in mice, and the apparent order of virulence is as follows: S2308 > S19 > RB51 (2, 28).
TABLE 1.
Protection of IRF-1−/− mice against S2308a
| B. abortus strain | Immunized CFU | % Protection (no. of animals protected/total no.) at:
|
||
|---|---|---|---|---|
| 4 wk p.c. | 8 wk p.c. | 12 wk p.c. | ||
| None (PBS) | 0 | 0 (0/10) | Dead | Dead |
| S19 | 5 × 101 | 80 (8/10) | 80 (8/10) | 80 (8/10) |
| S19 | 5 × 103 | 87.5 (7/8) | 87.5 (7/8) | 87.5 (7/8) |
| RB51 | 5 × 105 | 87.5 (7/8) | 87.5 (7/8) | 62.5 (5/8) |
| RB51 | 5 × 107 | 100 (9/9) | 100 (9/9) | 100 (9/9) |
| cyd mutant | 5 × 105 | 80 (8/10) | 60 (6/10) | 40 (4/10) |
| cyd mutant | 5 × 107 | 90 (9/10) | 90 (9/10) | 90 (9/10) |
IRF-1−/− mice were immunized with different Brucella strains. At 6 weeks after infection, mice in each group were challenged intraperitoneally with wild-type B. abortus 2308. p.c., postchallenge.
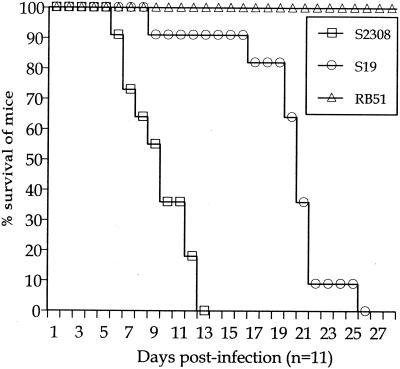
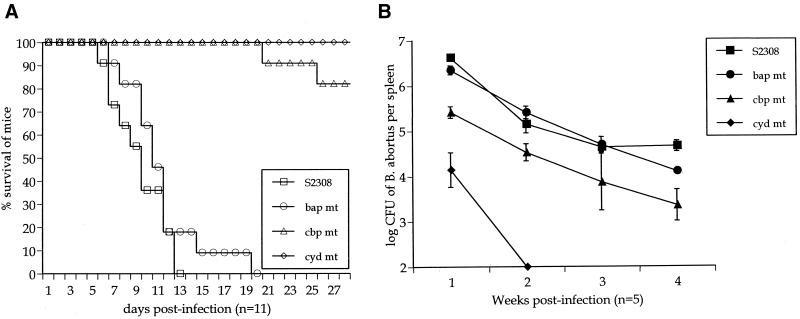
Fifty percent of IRF-1−/− mice injected with S2308 and S19 at 5 × 105 CFU died at 10 and 20 days postinoculation, respectively, whereas IRF-1−/− mice injected with the same dose of RB51 survived the 4 weeks of infection (Fig. 1). Thus, the life or death of the host induced by 5 × 105 CFU was dependent on Brucella virulence. Similarly, S2308 and S19 at 5 × 107 CFU produced death of 50% of IRF-1−/− mice between 6 to 7 days postinoculation, whereas the injection of the same CFU of RB51 failed to induce death of IRF-1−/− mice during the 4 weeks of infection (data not tabulated). All IRF-1−/− mice injected with 5 × 109 CFU of RB51 died 7 days later (data not tabulated). In contrast, IRF-1−/− mice injected with 5 × 101 or 5 × 103 CFU of S2308 and S19 survived infection (data not tabulated). Similarly, S2308 and S19 at 5 × 107 and RB51 at 5 × 109 CFU did not cause death of IRF-1+/+ mice (n = 3 per group) during the 4 weeks of infection (data not tabulated). Taken together, the death or survival of IRF-1−/− mice caused by respective doses of Brucella is a unique feature that may be related to the importance of the IRF-1 transcription factor in activating critical host protective mechanisms (19, 24). Also, these results indicate that the rapidity of death or the survival of IRF-1−/− mice is dependent on the dose and virulence of respective Brucella strains, and the injection of 5 × 105 CFU in IRF-1−/− mice can be an optimal bacterial concentration to differentiate the level of virulence in B. abortus strains. In fact, no RB51 (5 × 105 CFU)-infected mice died during the study, and 50% of S2308 infected mice were dead by day 10. The differences in the day postinfection when 50% of IRF-1−/− mice were dead could be a useful indicator to identify the degree of virulence (Table 1).
FIG. 1.
Susceptibility of IRF-1−/− mice (n = 11 per group) to B. abortus (5 × 105 CFU) S2308, S19, and RB51. Each symbol represents a day. IRF-1−/− mice infected with RB51 survived longer than S2308 (P < 0.0001)- or S19 (P < 0.0001)-infected mice. Mice infected with S19 survived longer than S2308 (P < 0.005)-infected mice.
Enumeration of B. abortus CFU from liver and spleen of S2308, S19, and RB51 infected IRF-1+/+ and IRF-1−/− mice.
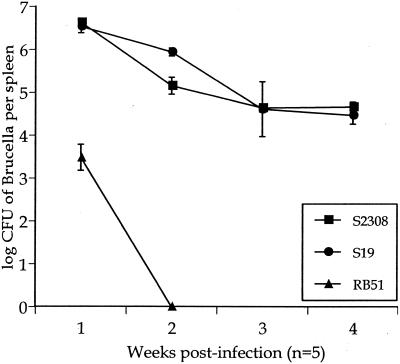
To correlate the level of virulence in B. abortus strains S2308, S19, and RB51 with the outcome of infection, IRF-1+/+ mice were infected with 5 × 105 CFU, and splenic Brucella CFU counts were determined for 4 weeks. Infected IRF-1+/+ mice had similar numbers of S2308 and S19 Brucella CFU during the 4 weeks of infection, although a statistically significant (P < 0.05) 1-log-higher amount of S19 was detected at 2 weeks postinoculation (Fig. 2). In contrast, RB51-infected IRF-1+/+ mice contained a 3-log-lower bacterial CFU in the spleen compared to S2308 and S19 infected mice after the first week of infection. In subsequent weeks, RB51 colonies were difficult to identify (Fig. 2).
FIG. 2.
Infection of IRF-1+/+ mice with 5 × 105 CFU of S2308, S19, and RB51. Five IRF-1+/+ mice were used for each time per group for the enumeration of splenic Brucella CFU.
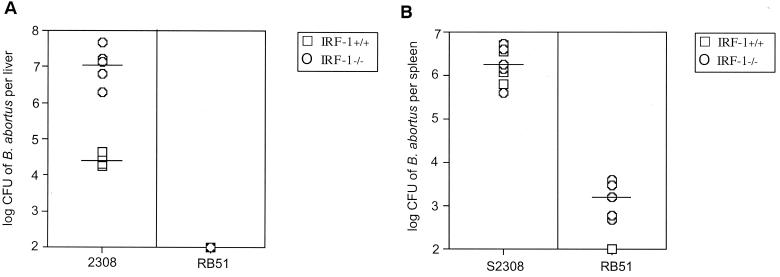
IRF-1−/− mice infected with virulent S2308 maintained a 1-log-greater bacterial CFU compared to infected IRF-1+/+ mice for the first 9 days, Fig. 3A. However, a 2-log difference in bacteria at day 12 was observed between IRF-1−/− and IRF-1+/+ mice in the liver (Fig. 3A) but not in the spleen (Fig. 3B). In contrast, IRF-1−/− and IRF-1+/+ mice infected with attenuated RB51 had a similar pattern of hepatic bacterial clearance, except that clearance in IRF-1−/− mice was delayed until day 12.
FIG. 3.
IRF-1−/− and IRF-1+/+ mice infected with B. abortus virulent S2308 and attenuated RB51 (5 × 105 CFU) strains for 12 days. The extent of bacteria recovered from liver (A) and spleen (B) indicated a 2.5-log reduction of S2308 from the livers of IRF-1+/+ mice compared to IRF-1−/− mice at day 12 (P < 0.0005). Clearance of RB51 occurred in the livers of IRF-1+/+ and IRF-1−/− mice.
Clearance of attenuated RB51 from the spleens of IRF-1−/− mice was more effective than clearance of virulent S2308 that persisted for the 12 days of infection. However, IRF-1−/− mice maintained RB51 at 3.5 to 4 log CFU in the spleen for the 12 days postinfection compared to the rapid reduction of bacteria in IRF-1+/+ mice (Fig. 3B).
Taken together, these results (Fig. 1 to 3) indicate that the degree of virulence was S2308 > S19 > RB51, as determined by CFU from the livers and spleens of IRF-1−/− mice. Also, virulence as determined by CFU correlated with the rapidity of death of infected IRF-1−/− mice in the order of S2308 > S19 > RB51.
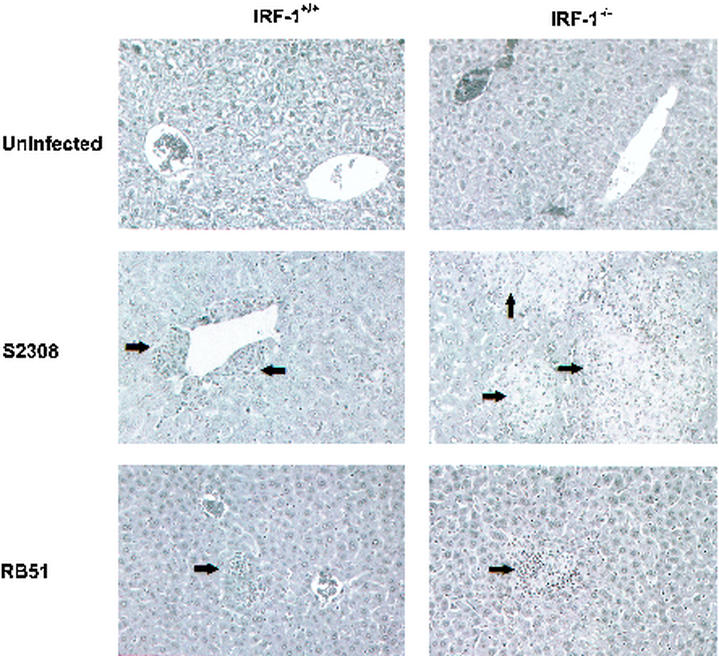
Histologic and phenotypic analysis of Brucella-infected IRF-1−/− and IRF-1+/+ mice.
Brucellosis induces chronic granuloma formation in the livers and spleens of humans and mice (5, 15a, 17). To determine whether pathologic changes could be detected with different strains of Brucella, IRF-1−/− and IRF-1+/+ mice were infected with 5 × 105 CFU of S2308 or RB51. Mice were killed at day 12, tissues were histologically examined, and consistent changes were noted in the livers. S2308 induced more granulomas in the livers of IRF-1−/− and IRF-1+/+ mice than RB51 did (Fig. 4). Further, more extensive multifocal granulomas were detected in IRF-1−/− mice compared to IRF-1+/+ mice (Fig. 4). These findings in IRF-1−/− mice indicate that the extent of liver damage caused by a B. abortus strain correlates with the hepatic CFU burden of B. abortus. Histologic analysis of brain, lung, heart, kidney, spleen, genital, and gastrointestinal organs did not indicate significant or consistent changes in these organs.
FIG. 4.
Histopathology of livers from IRF-1+/+ and IRF-1−/− mice infected with 5 × 105 CFU of S2308 or RB51. IRF-1−/− mice infected with S2308 had more extensive multifocal granulomas than infected IRF-1+/+ mice (arrows). RB51-infected IRF-1−/− and IRF-1+/+ mice had similar infrequent lymphoid aggregates.
Interestingly, infected moribund IRF-1−/− mice possessed a very unique phenotype visually discernible from healthy, infected IRF-1−/− mice (Fig. 5). IRF-1−/− mice infected with B. abortus S2308 or S19 at 5 × 105 CFU contained 6 to 10 ml of ascitic fluid, whereas the RB51 infected IRF-1−/− mice did not. These findings in IRF-1−/− mice suggest that virulent Brucella infection causes hepatic damage, leading to ascites production, and the acquisition of ascitic fluid could be a key clinical sign to detect the level of virulence in Brucella strains.
FIG. 5.
Ascites of 5 × 105 CFU S2308-infected IRF-1−/− mice at day 12 compared to RB51-infected mice. A total of 6 to 10 ml of fluid was typically in the abdomen. Cells in the fluid were >90% macrophages.
Survival of IRF-1+/+ and IRF-1−/− mice infected with cyd, cbp, and bap S2308 mutants.
The survival or death of IRF-1−/− mice infected with 5 × 105 CFU of S2308, S19, or RB51 (Fig. 1) and residual hepatic and splenic CFU in IRF-1−/− mice (Fig. 3) suggest that IRF-1−/− mice may be useful for evaluating the role in bacterial virulence of selected bacterial genes. Using gene-deleted or gene-interrupted mutant bacteria, the importance of a given gene in bacterial virulence can be determined. To test this possibility, three Brucella mutants (cyd, cbp, and bap) were introduced. cyd (9) and cbp (Ficht, unpublished) are gene-interrupted mutants of cytochrome d oxidase and a calcium-binding protein, respectively, whereas bap (15) is a gene-deletion mutant of an ATP-binding protein.
Importantly, when IRF-1−/− mice were infected with the B. abortus cbp and cyd mutants, 5 × 105 CFU of the cyd mutant did not induce the death of IRF-1−/− mice, but the cbp mutant produced two deaths from 11 infected mice after 20 days postinoculation (Fig. 6A). Thus, the degree of attenuation in the cbp mutant was not sufficient to prevent the death of IRF-1−/− mice. In IRF-1−/− mice, bap mutants induced death in 50% of the mice 12 days after infection (Fig. 6A) compared to S2308 infection that induced the death of 50% of IRF-1−/− mice at 10 days. However, this discrepancy was not statistically significant.
FIG. 6.
(A) Survival of IRF-1−/− mice (n = 11) infected with mutant Brucella (bap, cbp, and cyd) or S2308. Mice infected with cyd or cbp mutants survived longer than S2308-infected mice (P < 0.0001). However, bap mutant infection was not statistically significant. (B) Growth of mutant Brucella (bap, cbp, and cyd) compared to S2308 in the spleens of infected IRF-1+/+ mice. Five IRF-1+/+ mice were used for each time per group. Each symbol represents a day.
Taken together, these results indicate that IRF-1−/− mice infected with cyd or cbp mutants survived longer than when infected with S2308 (P < 0.0001). IRF-1−/− mice infected with the bap mutant survived as long as mice infected with S2308. Thus, the level of virulence from Brucella mutants, as reflected in CFU in the spleens of IRF-1+/+ mice, is compatible with virulence as defined by the rapidity of death in IRF-1−/− mice infected with 5 × 105 CFU of S2308, S19, or RB51.
Enumeration of CFU from liver and spleen of IRF-1+/+ and IRF-1−/− mice infected with cyd, cbp, and bap S2308 mutants.
Infection of IRF-1+/+ and IRF-1−/− mice with S2308 revealed a persistent increase in bacterial CFU in the livers of IRF-1−/− mice compared to those of IRF-1+/+ mice (Fig. 3).
To determine the clearance of cyd, cbp, and bap S2308 mutants from mice, splenic B. abortus CFU were first counted from IRF-1+/+ mice after infection with these mutants at 5 × 105 CFU (Fig. 6B). The cyd gene-interrupted mutant was rarely detected in spleens after 2 weeks postinfection from the spleens. CFU detected from mice infected with the cbp gene-interrupted mutant had a 1-log difference compared to S2308-infected mice during the 4 weeks of infection. The parental S2308 and cbp mutant had statistically different (P < 0.05) CFU at all weeks examined (Fig. 6B). In contrast to cyd and cbp mutants, no significant difference in CFU of bacteria from spleens was detected between the bap mutant and its parental strain during the 4 weeks of infection in IRF-1+/+ mice. Thus, the apparent order of virulence detected by splenic CFU in IRF-1+/+ mice was S2308 = bap > cbp > cyd = RB51.
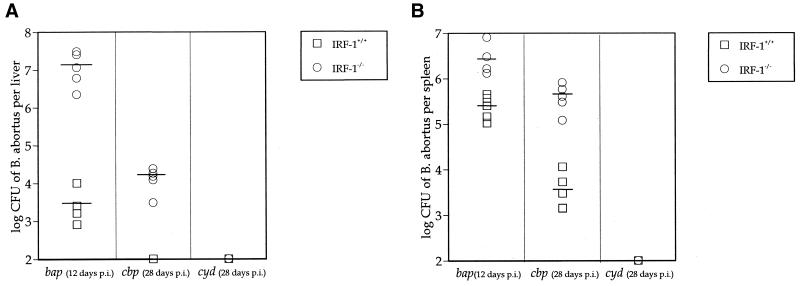
To determine whether hepatic damage from bap, cbp, and cyd mutants correlated with the criteria for virulence with S2308, S19, and RB51 in IRF-1−/− mice, IRF-1−/− and IRF-1+/+ mice were infected with these mutants, and Brucella CFU were enumerated from livers (Fig. 7A) and spleens (Fig. 7B) at the moribund stage or 4 weeks after infection. Twelve days after bap mutant injection, a 3.5-log CFU difference in livers and a 1-log CFU difference in spleens was detected between IRF-1+/+ and IRF-1−/− mice. These results coincide with results in Fig. 3 with S2308-infected IRF-1+/+ and IRF-1−/− mice, confirming that hepatic bacterial burden correlates with the death of IRF-1−/− mice. At 4 weeks after cbp mutant injection, 4 logs of bacterial CFU were detected in the livers of IRF-1−/− mice, whereas no B. abortus were detected from the livers of IRF-1+/+ mice (Fig. 7A), suggesting that residual basal immunity in IRF-1−/− mice may control cbp infection. Additionally, a 2-log CFU difference in the cbp mutant was detected from the spleens of IRF-1+/+ and IRF-1−/− mice (Fig. 7B). The hepatic bacterial burden induced by cbp mutants in IRF-1−/− mice at 4 weeks after infection was 3 logs less than the CFU detected from bap- or S2308-infected IRF-1−/− mice at the moribund stage. No cyd mutant was detected from the livers or spleens of IRF-1+/+ or IRF-1−/− mice at 4 weeks after infection. Therefore, these results support a correlation between hepatic bacterial burden and the death of IRF-1−/− mice and suggest that a level of virulence equivalent to that of S2308 is required to produce death in IRF-1−/− mice.
FIG. 7.
Hepatic (A) and splenic (B) Brucella CFU from IRF-1−/− mice compared to IRF-1+/+ mice infected with B. abortus cbp, cyd, and bap mutants (5 × 105 CFU). Mice were at the moribund stage or 4 weeks postinoculation. Hepatic bap CFU detected from IRF-1+/+ and IRF-1−/− mice showed a ∼3.5-log difference at 12 days postinoculation (P < 0.005), indicating the serious bacterial burden in IRF-1−/− mice. No difference in bacterial growth was noted in the spleens of IRF-1+/+ and IRF-1−/− mice. However, attenuated cbp and cyd mutants were controlled by IRF-1−/− mice at 4 weeks postinoculation.
Biochemical analysis of hepatic damage in IRF-1−/− mice.
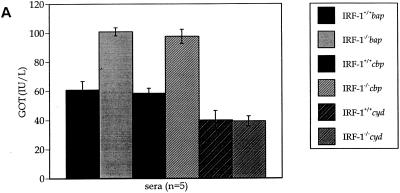
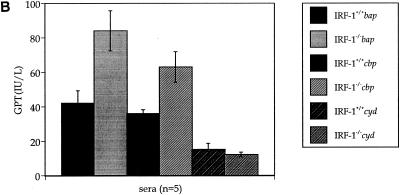
The existence of ascitic fluid (Fig. 5), as well as the hepatic bacterial burden in IRF-1−/− mice infected with virulent Brucella strains (Fig. 6A), suggests progression of liver damage. Because livers from IRF-1−/− mice infected with B. abortus strain S2308 had marked granulomas (Fig. 4), GOT, GPT, and albumin concentrations in sera were measured to quantify the level of hepatic damage at the moribund stage or 4 weeks postinoculation (Fig. 8). Both uninfected IRF-1+/+ and IRF-1−/− mice maintained a normal GOT and GPT activity in serum (37 and 19 IU/liter, respectively [data not shown]) (23). Also, IRF-1+/+ and IRF-1−/− mice infected with cyd mutants did not have increased GOT and GPT activities in sera at 4 weeks postinoculation. In contrast, bap- and cbp-infected IRF-1+/+ and IRF-1−/− mice possessed increased GOT (Fig. 8A) and GPT (Fig. 8B) activities at the moribund stage, indicating that acute hepatitis occurred in both mouse strains. However, the extent of liver damage between the two mouse strains was evident with a twofold increase in IRF-1+/+ mice and a fourfold-increased GOT and GPT levels in IRF-1−/− mice. These results suggest that 4 weeks of cbp mutant infection generates hepatic damage similar to that induced by S2308 (Fig. 3 and 4) or bap mutants at 12 days postinoculation, suggesting that the death of cbp-infected IRF-1−/− mice may occur after 4 weeks postinoculation. In fact, two more mice were dead after the 4-week study, and many mice were not healthy (data not shown). Taken together, these GOT and GPT results indicate virulence-dependent hepatic damage occurred in both mouse strains, but the level of hepatic damage in IRF-1−/− mice was more severe than in IRF-1+/+ mice.
FIG. 8.
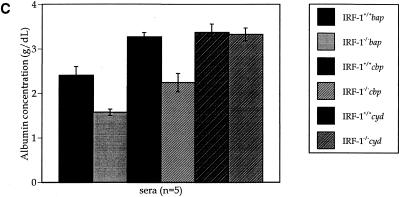
GOT (A) and GPT (B) activity and albumin concentration (C) assays. Sera were collected from IRF-1+/+ and IRF-1−/− mice infected with cbp, cyd, and bap mutants (5 × 105 CFU) at the moribund stage or 4 weeks postinoculation. The levels of increased GOT and GPT activities and decreased albumin concentration in sera from bap or cbp mutant-infected IRF-1−/− mice were discernible from bap or cbp mutant-infected IRF-1+/+ mice and cyd-infected IRF-1−/− mice, indicating serious liver damage in bap mutant-infected IRF-1−/− mice at the moribund stage or in cbp mutant-infected IRF-1−/− mice at 4 weeks postinoculation.
The existence of ascitic fluid (6 to 10 ml/mouse) in IRF-1−/− mice infected with the bap mutant suggests that the concentration of albumin in sera may have been low since albumin is an osmotic regulator produced by the liver (13). Both uninfected (data not shown) and cyd-infected IRF-1+/+ and IRF-1−/− mice maintained normal levels of serum albumin (3.4 g/dl; Fig. 8C) (23). However, bap mutant-infected IRF-1+/+ and IRF-1−/− mice had a 29 and a 53% reduction in albumin, indicating that the disruption of osmotic regulation occurred in both mouse strains. cbp mutant-infected IRF-1+/+ and IRF-1−/− mice showed a 35% reduction in albumin only in IRF-1−/− mice (Fig. 8C).
As a result, GOT and GPT, as well as albumin, assays identified serious liver damage and disruption of osmotic regulation in IRF-1−/− mice infected with the bap mutant at the moribund stage, less-serious liver damage in IRF-1−/− mice infected with the cbp mutant at 4 weeks, and no hepatic damage in IRF-1−/− mice infected with the cyd mutant at 4 weeks postinoculation. These results suggest that death in IRF-1−/− mice involves liver damage and that a certain level of bacterial virulence is required to induce liver damage in IRF-1−/− mice.
Brucella dose- and virulence-dependent protection in immunized IRF-1−/− mice.
IRF-1−/− mice were immunized with selected Brucella strains at sublethal doses for 6 weeks and challenged with the lethal dose (5 × 105 CFU) of S2308. All IRF-1−/− mice immunized with any Brucella strain survived longer than nonimmunized IRF-1−/− mice, suggesting that immunological memory exists and can provide protection (Table 2). In addition, two major results were noticed. First, in terms of Brucella strains possessing the same level of virulence, higher doses of immunization provide better protection. For example, IRF-1−/− mice immunized with RB51 or cyd mutants at 5 × 107 CFU provided twofold greater protection than mice immunized with 5 × 105 CFU. Second, the more attenuated the Brucella strain, the higher the dose of immunization required to provide protection. For instance, IRF-1−/− mice immunized with S19 at 5 × 103 CFU provided similar protection to IRF-1−/− mice immunized with RB51 or cyd mutants at 5 × 107 CFU. These results indicate that IRF-1−/− mice maintain an adaptive immunological memory necessary for protection, and protection in IRF-1−/− mice is dependent on the level of bacterial virulence and the dose of immunization.
TABLE 2.
Criteria for Brucella strain virulence in IRF-1−/− mice
| Criterion |
Brucella strain virulence
|
||
|---|---|---|---|
| Low | Intermediate | High | |
| Days of survivala | RB51, cyd | S19, cbp | S2308, bap |
| Liver featuresb | RB51, cyd | S19, cbp | S2308, bap |
| Protectionc | RB51, cyd | S19 | S2308 |
That is, the day when 50% of the mice were alive after infection with 5 × 105 CFU of a Brucella strain. Fifty percent of mice surviving for 30 days is low, 19 to 25 days is intermediate, and < 18 days is high virulence.
Combined changes in albumin, hepatic GOT, GPT, and hepatic granuloma formation.
Survival of vaccinated IRF-1−/− mice for 6 weeks after challenge with S2308 at 5 × 105 CFU. Low virulence is >90% of mice surviving, intermediate virulence is 60 to 89% of mice surviving, and high virulence is <50% of mice surviving.
DISCUSSION
Human brucellosis is a chronic, undulating disease, and currently there is no human vaccine. Thus, there is a need to rank the virulence of mutant Brucella that might serve as potential vaccine candidates. Mice have been used to determine Brucella virulence since the chronic nature of Brucella infection in mice parallels the chronic nature of human infection (3, 20). However, such studies are complicated by the need to monitor bacterial clearance from organs for at least 6 weeks, requiring large numbers of mice to frequently sample for bacteria, and such experiments are labor-intensive. Therefore, animal models that provide a rapid measure of Brucella virulence are needed.
The IRF-1−/− mouse provides a useful set of three criteria used to define virulence in brucellosis; these are summarized in Table 1. First, the day when 50% of IRF-1−/− mice die after injection of 5 × 105 CFU of bacteria is an important indication of virulence. The more rapidly the death occurs, the greater the bacterial virulence. Second, the extent of liver damage, e.g., the presence of granulomas, the liver transaminase activity, and the hepatic CFU bacterial count supports the virulence of the Brucella strain. Third, the minimum immunizing dose for protection against challenge with virulent S2308 (5 × 105 CFU) indicates the decreased virulence of the immunizing strain, as well as its ability to induce immune protection.
IRF-1−/− mice provide a new animal model to analyze the contribution of individual Brucella genes to bacterial virulence. Using several Brucella gene-deleted or gene-interrupted mutants, we applied these virulence criteria to three engineered mutant Brucella to evaluate the contribution of individual bacterial genes to virulence. Thus, bacterial CFU in spleens and livers as well as the length of survival of IRF-1−/− mice infected with different brucellae can be used to dissect the mechanisms of virulence as a component of bacterial pathogenesis.
Our initial observation regarding the acute death of IRF-1−/− mice by strain S2308 but not by strain RB51 suggests that IRF-1−/− mice respond to B. abortus strains differently depending on the level of virulence in each strain (15a). Thus, a certain level of bacterial virulence is required to induce the death of IRF-1−/− mice, and/or a basal level of immunity remains in IRF-1−/− mice sufficient to control infection caused by an attenuated bacterial strain such as RB51. Therefore, we explored bacterial pathogenesis with diverse Brucella strains containing various levels of virulence in IRF-1−/− mice.
Three different experiments were performed in IRF-1−/− mice to investigate each of three criteria for virulence. First, dose-dependent responses of IRF-1−/− mice against B. abortus S2308, S19, and RB51 strains were investigated, and 5 × 105 CFU was identified as an optimized amount to differentiate IRF-1−/− mouse survival or death induced by a Brucella strain (Fig. 1) and bacterial clearance from organs (Fig. 2). Second, using genetically identified bap, cbp, and cyd B. abortus mutants containing distinctive levels of persistence in vitro (15) and in vivo (9, 15), the application of IRF-1−/− mice to detect virulence was tested (Fig. 4). Third, the correlation between the level of virulence and the level of hepatic damage was determined by measuring the hepatic bacterial CFU and transaminase activities, as well as the albumin concentration in serum at either the moribund stage or 4 weeks postinoculation, suggesting that death in IRF-1−/− mice is associated with hepatic damage (Fig. 3, 5, and 6). Together, these results suggest that the level of Brucella virulence can be determined by IRF-1−/− mouse survival and that the level of Brucella virulence correlates with bacterial CFU and level of hepatic damage, as reflected by transaminase and albumin levels in serum.
One manifestation of human brucellosis is acute hepatitis (8, 17). Most human beings or animals infected with Brucella overcome this acute hepatitis and maintain a chronic infection, and this transition is dependent on the virulence of Brucella strains and the induction of an adaptive immune response (4, 17). However, in some cases, human brucellosis results in death. For example, humans with Brucella peritonitis and liver problems, such as alcoholism or hepatitis virus infection, contain ascites and can die if proper treatment does not follow (1, 5, 22, 30). Similarly, in IRF-1−/− mice in which hepatitis occurs in the absence of important adaptive immune components, animals infected with virulent strain 2308 are unable to control infection. IRF-1−/− mice infected with the virulent strain died within 12 days, supporting the role of IRF-1 as essential for host immunity to Brucella; however, the defective immune components in IRF-1−/− mice that contribute to Brucella susceptibility are unknown. In IRF-1−/− mice, the host immune mechanisms that fail to control hepatic damage caused by virulent Brucella require further experiments.
In contrast to lethality when IRF-1−/− mice were infected with Brucella S2308, IRF-1−/− mice infected with attenuated Brucella strains did not die, supporting the possibility that IRF-1−/− mice have a level of adaptive immunity that may protect these mice. Additional studies are required to confirm the relationship between Brucella virulence and dose-dependent immune protection in IRF-1−/− mice. Survival of IRF-1−/− mice was unexpected since this mouse strain is deficient in IL-12 p40 induction that is required for a Th1 immune response against many intracellular pathogens (18, 29). We hypothesize Kupffer cells may not control virulent Brucella because the levels of IFN-γ and TNF-α in IRF-1−/− mice are lower than in wild-type mice (26). Insufficient levels of IFN-γ and TNF-α may not activate phagocytic activity of Kupffer cells, permitting continued B. abortus proliferation in the liver.
The death of IRF-1−/− mice after virulent Brucella infection is a unique feature in brucellosis because even SCID (21) and nude (6) mice control Brucella infection. IRF-1−/− mice are deficient in CD8+ T cells and NK cells, as well as in the induction of IL-12, p40, and iNOS (18, 29). This phenotypically well-characterized mouse strain has been used to investigate the role of IRF-1 or the role of defective immune components during infection. For example, IRF-1−/− mice are susceptible to Mycobacterium bovis BCG (12), Leishmania major (16), and Toxoplasma gondii (14) due to the absence of iNOS induction and/or IL-12 p40 induction but are resistant to Listeria monocytogenes (11) infection. The death of IRF-1−/− mice infected with T. gondii has been reported previously, but the dose- and virulence-dependent death and the cause of death have not been investigated. Thus, the susceptibility of IRF-1−/− mice appears to be pathogen specific.
IRF-1−/− mice can serve as a useful animal for evaluating mutant brucellae. We have identified Brucella promoters and associated genes activated early in macrophage infection (10). Now, using targeted gene disruption, IRF-1−/− mice can be used to determine the association of these bacterial genes with virulence in the process of bacterial pathogenesis.
In summary, we have demonstrated that virulence can be defined by (i) the rapidity of death in IRF-1−/− mice infected with 5 × 105 CFU; (ii) the level of hepatic damage detected by the increased hepatic bacterial burden, as well as transaminase and albumin assays; and (iii) dose-dependent immune protection against virulent Brucella challenge can be engendered in IRF-1−/− mice by attenuated Brucella strains. These results suggest that the application of IRF-1−/− mice to brucellosis research will aid in the understanding of this host-pathogen interaction. Further, IRF-1−/− mice, as an animal model, provide a rapid assessment of Brucella virulence that is useful for screening large numbers of Brucella strains. In addition, IRF-1−/− mice will contribute to defining the adaptive immune components and molecular mechanisms of immunity required for protection. Defined criteria for virulence combined with a rapid screening mouse model, the IRF-1−/− mouse, will facilitate the development of novel vaccines.
Acknowledgments
We thank Tak W. Mak at University of Toronto for providing breeding pairs of IRF-1−/− mice and Peter S. MacWilliams at University of Wisconsin for discussing serum transaminase and albumin assays.
This work was supported by USDA grant 98-35204-6760, Binational Agricultural Research and Development Fund grants US-2781-96 and US-2968-98C, and NIH grant R01-AI48490. T.A.F. was supported by a grant from USDA/CSREES 92-37204-8002.
Editor: V. J. DiRita
REFERENCES
- 1.Alcala, L., P. Munoz, M. Rodriguez-Creixems, R. Banares, and E. Bouza. 1999. Brucella spp. peritonitis. Am. J. Med. 107:300. [PubMed] [Google Scholar]
- 2.Baldwin, C. L., and A. J. Winter. 1994. Macrophages and Brucella. Immunol. Ser. 60:363-380. [PubMed] [Google Scholar]
- 3.Bosseray, N., and Plommet, M. 1990. Brucella suis S2, Brucella melitensis Rev.1 and Brucella abortus S19 living vaccines: residual virulence and immunity induced against three Brucella species challenge strains in mice. Vaccine 8:462.. [DOI] [PubMed] [Google Scholar]
- 4.Brinley-Morgan, W. J., and M. J. Corbel. 1990. Brucella infections in man and animals; contagious equine metritis, p. 547-570. In Topley and Wilson's principles of bacteriology, virology and immunology, 8th ed. Edward Arnold, London, England.
- 5.Caballeria, E., R. M. Masso, J. V. Arago, and A. Sanchis. 1992. Ascites as the first manifestation of Brucella granulomatous hepatitis. J. Hepatol. 15:415-416. [DOI] [PubMed] [Google Scholar]
- 6.Cheville, N. F., R. A. Kunkle, A. E. Jensen, and M. V. Palmer. 1995. Persistence of Brucella abortus in the livers of T cell-deficient nude mice. Lab. Investig. 73:96-102. [PubMed] [Google Scholar]
- 7.Corbel, M. J. 1990. Brucella, p. 339-353. In Topley and Wilson's principles of bacteriology, virology, and immunology, 8th ed. Edward Arnold, London, England.
- 8.Crawford, J. M. 1999. The liver and the biliary tract, p. 845-901. In R. S. Cotran, V. Kurmar, and T. Collins (ed.), Robbins pathologic basis of disease, 6th ed. The W. B. Saunders Company, Philadelphia, Pa.
- 9.Endley, S., D. McMurray, and T. A. Ficht. 2001. Interruption of the cydB locus in Brucella abortus attenuates intracellular survival and virulence in the mouse model of infection. J. Bacteriol. 183:2454-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eskra, L., A. Canavessi, M. Carey, and G. Splitter. 2001. Brucella abortus genes identified following constitutive growth and macrophage infection. Infect. Immun. 69:7736-7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fehr, T., G. Schoedon, B. Odermatt, T. Holtschke, M. Schneemann, M. F. Bachmann, T. W. Mak, I. Horak, and R. M. Zinkernagel. 1997. Crucial role of interferon consensus sequence binding protein, but neither of interferon regulatory factor 1 nor of nitric oxide synthesis for protection against murine listeriosis. J. Exp. Med. 185:921-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamijo, R., H. Harada, T. Matsuyama, M. Bosland, J. Gerecitano, D. Shapiro, J. Le, S. I. Koh, T. Kimura, and S. J. Green. 1994. Requirement for transcription factor IRF-1 in NO synthase induction in macrophages. Science 263:1612-1615. [DOI] [PubMed] [Google Scholar]
- 13.Kaneko, J. J. 1989. Serum proteins and the dysproteinemias, p. 142-165. In J. J. Kaneko (ed.), Clinical biochemistry of domestic animals, 4th ed. Academic Press, San Diego, Calif.
- 14.Khan, I. A., T. Matsuura, S. Fonseka, and L. H. Kasper. 1996. Production of nitric oxide (NO) is not essential for protection against acute Toxoplasma gondii infection in IRF-1−/− mice. J. Immunol. 156:636-643. [PubMed] [Google Scholar]
- 15.Ko, J., and G. A. Splitter. 2000. Brucella abortus tandem repeated ATP-binding proteins, BapA and BapB, homologs of Haemophilus influenzae LktB, are not necessary for intracellular survival. Microb. Pathog. 29:245-253. [DOI] [PubMed] [Google Scholar]
- 15a.Ko, J., A. Gendron-Fitzpatrick, and G. Splitter. 2002. Susceptibility of interferon regulatory factor-1 (IRF-1) and interferon consensus sequence binding protein (ICSBP) deficient mice to brucellosis. J. Immunol. 168:2433-2440. [DOI] [PubMed] [Google Scholar]
- 16.Lohoff, M., D. Ferrick, H. W. Mittrucker, G. S. Duncan, S. Bischof, M. Rollinghoff, and T. W. Mak. 1997. Interferon regulatory factor-1 is required for a T helper 1 immune response in vivo. Immunity 6:681-689. [DOI] [PubMed] [Google Scholar]
- 17.Madkour, M. M. 2001. Madkour's brucellosis, p. 150-151. Springer-Verlag, New York, N.Y.
- 18.Mamane, Y., C. Heylbroeck, P. Genin, M. Algarte, M. J. Servant, C. LePage, C. DeLuca, H. Kwon, R. Lin, and J. Hiscott. 1999. Interferon regulatory factors: the next generation. Gene 237:1-14. [DOI] [PubMed] [Google Scholar]
- 19.Matsuyama, T., T. Kimura, M. Kitagawa, K. Pfeffer, T. Kawakami, N. Watanabe, T. M. Kundig, R. Amakawa, K. Kishihara, and A. Wakeham. 1993. Targeted disruption of IRF-1 or IRF-2 results in abnormal type I IFN gene induction and aberrant lymphocyte development. Cell 75:83-97. [PubMed] [Google Scholar]
- 20.Montaraz, J. A., and Winter, A. J. 1986. Comparison of living and nonliving vaccines for Brucella abortus in BALB/c mice. Infect. Immun. 53:245.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morfitt, D. C., N. F. Cheville, and A. E. Jensen. 1991. Diminution of virulence of B. abortus for severe combined immunodeficiency mice, p. 2. In Proceedings of the Conference of Research Workers in Animal Diseases, vol. 72. Iowa State University Press, Ames, Iowa.
- 22.Ozakyol, A. H., T. Saricam, and I. Zubaroglu. 1999. Spontaneous bacterial peritonitis due to Brucella melitensis in a cirrhotic patient. Am. J. Gastroenterol. 94:2572-2573. [DOI] [PubMed] [Google Scholar]
- 23.Quinn, F. D. 1999. Mouse, p. 3-32. In W. F. Loeb and F. W. Quimby (ed.), The clinical chemistry of laboratory animals, 2nd ed. Taylor & Francis, Philadelphia, Pa.
- 24.Salkowski, C. A., S. A. Barber, G. R. Detore, and S. N. Vogel. 1996. Differential dysregulation of nitric oxide production in macrophages with targeted disruptions in IFN regulatory factor-1 and -2 genes. J. Immunol. 156:3107-3110. [PubMed] [Google Scholar]
- 25.Sangari, F. J., and J. Aguero. 1996. Molecular basis of Brucella pathogenicity: an update. Microbiologia 12:207-218. [PubMed] [Google Scholar]
- 26.Senaldi, G., C. L. Shaklee, J. Guo, L. Martin, T. Boone, W. Mak, and T. R. Ulich. 1999. Protection against the mortality associated with disease models mediated by TNF and IFN-γ in mice lacking IFN regulatory factor-1. J. Immunol. 163:6820-6826. [PubMed] [Google Scholar]
- 27.Splitter, G., S. Oliveira, M. Carey, C. Miller, J. Ko, and J. Covert. 1996. T lymphocyte mediated protection against facultative intracellular bacteria. Vet. Immunol. Immunopathol. 54:309-319. [DOI] [PubMed] [Google Scholar]
- 28.Stevens, M. G., S. C. Olsen, G. W. Pugh, Jr., and M. V. Palmer. 1994. Immune and pathologic responses in mice infected with Brucella abortus 19, RB51, or 2308. Infect. Immun. 62:3206-3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taniguchi, T., K. Ogasawara, A. Takaoka, and N. Tanaka. 2001. IRF family of transcription factors as regulators of host defense. Annu. Rev. Immunol. 19:623-655. [DOI] [PubMed] [Google Scholar]
- 30.Yumuk, Z., S. Ozdemirci, B. F. Erden, and V. Dundar. 2001. The effect of long-term ethanol feeding on Brucella melitensis infection of rats. Alcohol Alcohol. 36:314-317. [DOI] [PubMed] [Google Scholar]