Abstract
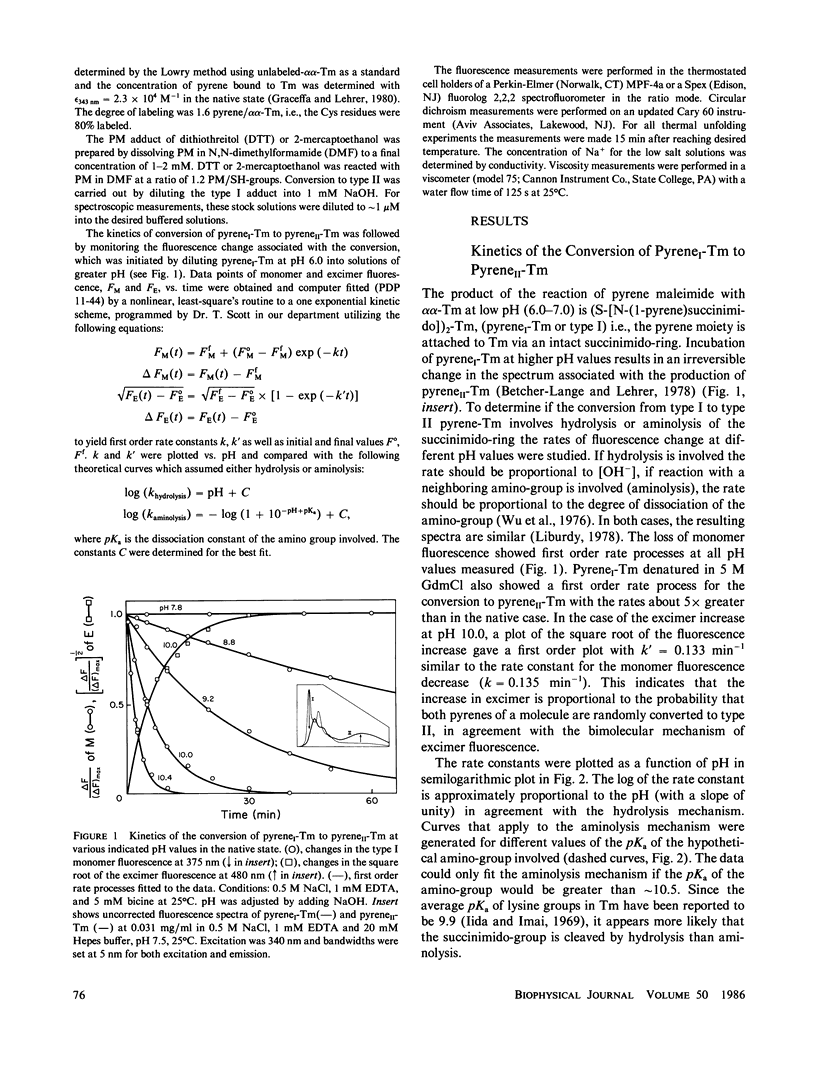
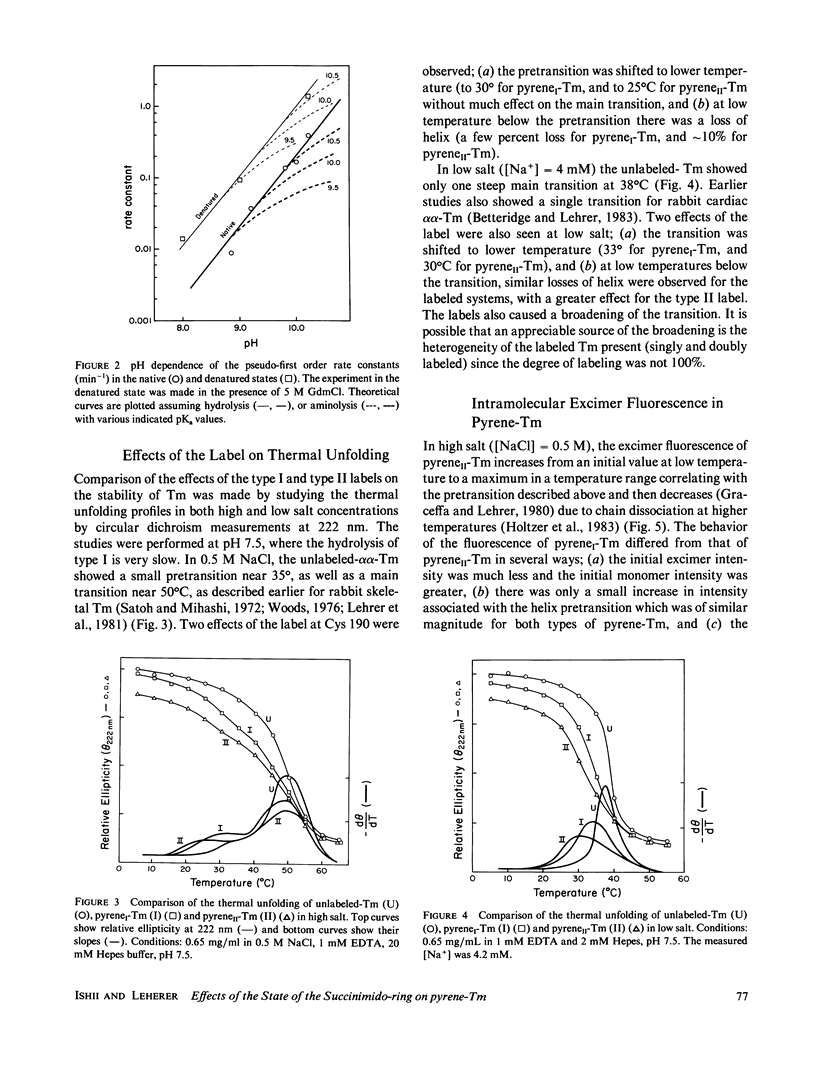
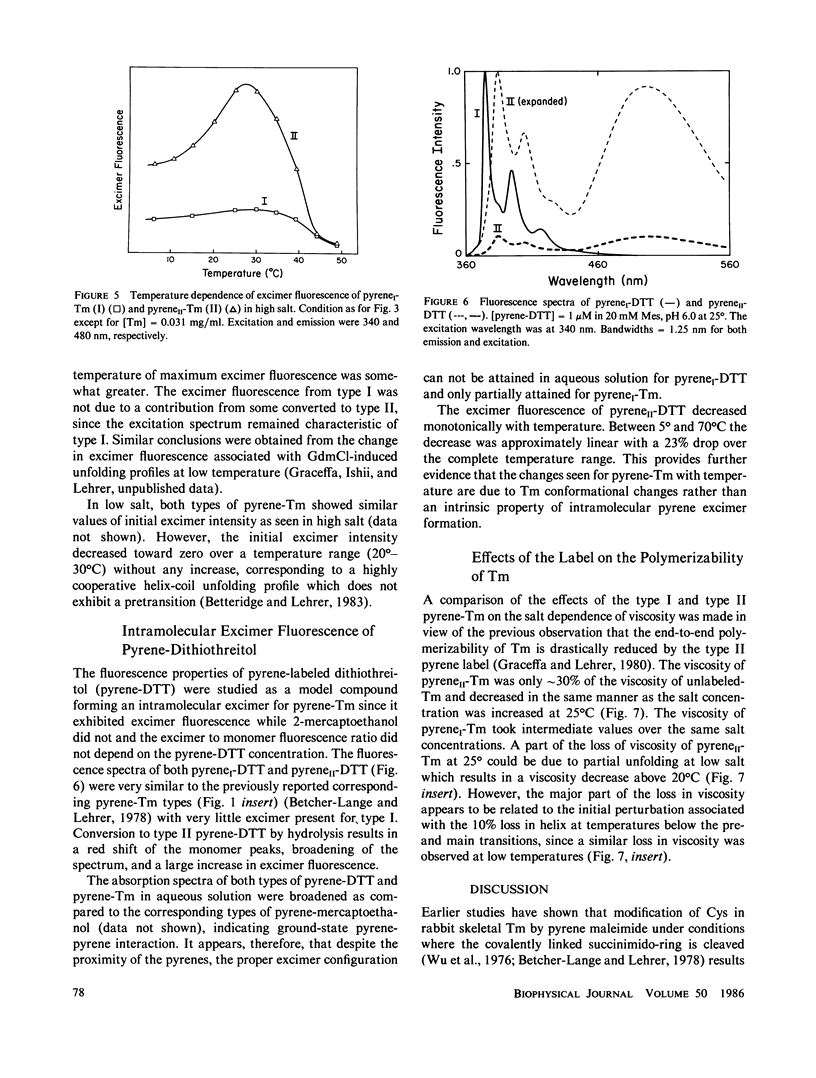
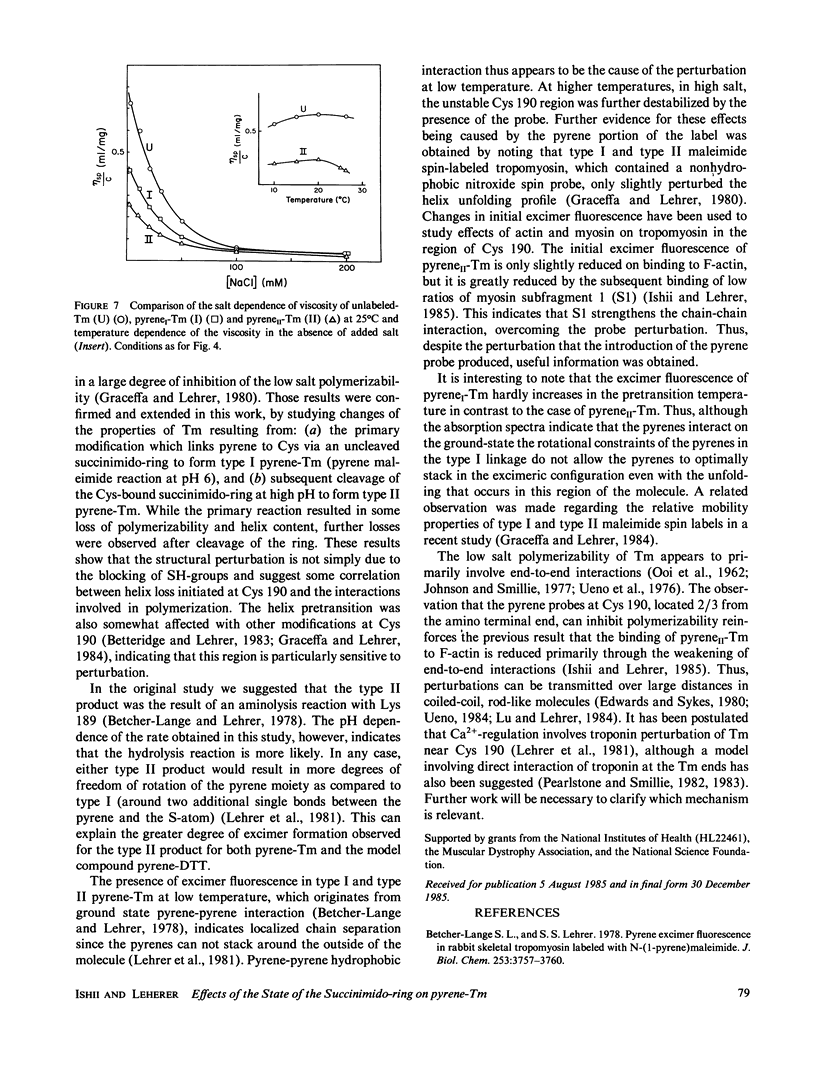
Rabbit skeletal alpha alpha-tropomyosin was labeled at Cys-190 with pyrene maleimide to form (S-[N-(1-pyrene)succinimido])2-tropomyosin (pyreneI-Tm). The product with cleaved succinimido-rings, pyreneII-Tm was also prepared by incubation of pyreneI-Tm at pH greater than 7.5. The pH dependence of the rate of cleavage indicated that hydrolysis rather than aminolysis was the more likely reaction. PyreneI-Tm exhibited a loss in helix content and end-to-end polymerization compared with unlabeled Tm, which increased upon formation of pyreneII-Tm. The cleavage resulted in increased interchain excited state excimer fluorescence originating from pyrene-pyrene interaction between the chains. Thus, increased pyrene-pyrene interaction at Cys 190 leads to an increase in unfolding, the effects of which appear to be transmitted to the ends of tropomyosin. The fluorescence properties of the two types of pyrene-succinimide adducts of dithiothreitol were very similar to the corresponding adducts of pyrene-Tm indicating excimer formation through ground state pyrene-pyrene interaction.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betcher-Lange S. L., Lehrer S. S. Pyrene excimer fluorescence in rabbit skeletal alphaalphatropomyosin labeled with N-(1-pyrene)maleimide. A probe of sulfhydryl proximity and local chain separation. J Biol Chem. 1978 Jun 10;253(11):3757–3760. [PubMed] [Google Scholar]
- Betteridge D. R., Lehrer S. S. Two conformational states of didansylcystine-labeled rabbit cardiac tropomyosin. J Mol Biol. 1983 Jun 25;167(2):481–496. doi: 10.1016/s0022-2836(83)80346-5. [DOI] [PubMed] [Google Scholar]
- Cummins P., Perry S. V. The subunits and biological activity of polymorphic forms of tropomyosin. Biochem J. 1973 Aug;133(4):765–777. doi: 10.1042/bj1330765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Edwards B. F., Sykes B. D. Nuclear magnetic resonance evidence for the coexistence of several conformational states of rabbit cardiac and skeletal tropomyosins. Biochemistry. 1980 Jun 10;19(12):2577–2583. doi: 10.1021/bi00553a007. [DOI] [PubMed] [Google Scholar]
- Graceffa P., Lehrer S. S. Dynamic equilibrium between the two conformational states of spin-labeled tropomyosin. Biochemistry. 1984 Jun 5;23(12):2606–2612. doi: 10.1021/bi00307a011. [DOI] [PubMed] [Google Scholar]
- Graceffa P., Lehrer S. S. The excimer fluorescence of pyrene-labeled tropomyosin. A probe of conformational dynamics. J Biol Chem. 1980 Dec 10;255(23):11296–11300. [PubMed] [Google Scholar]
- Greene L. E., Eisenberg E. Cooperative binding of myosin subfragment-1 to the actin-troponin-tropomyosin complex. Proc Natl Acad Sci U S A. 1980 May;77(5):2616–2620. doi: 10.1073/pnas.77.5.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L., Eisenberg E., Chalovich J. M. Theoretical models for cooperative steady-state ATPase activity of myosin subfragment-1 on regulated actin. Biophys J. 1981 Jul;35(1):99–112. doi: 10.1016/S0006-3495(81)84777-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L., Eisenberg E., Greene L. Theoretical model for the cooperative equilibrium binding of myosin subfragment 1 to the actin-troponin-tropomyosin complex. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3186–3190. doi: 10.1073/pnas.77.6.3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida S., Imai N. Hydrogen ion titration and sodium ion activity of tropomyosin solutions. J Phys Chem. 1969 Jan;73(1):75–80. doi: 10.1021/j100721a013. [DOI] [PubMed] [Google Scholar]
- Ishii Y., Lehrer S. S. Fluorescence studies of the conformation of pyrene-labeled tropomyosin: effects of F-actin and myosin subfragment 1. Biochemistry. 1985 Nov 5;24(23):6631–6638. doi: 10.1021/bi00344a050. [DOI] [PubMed] [Google Scholar]
- Johnson P., Smillie L. B. Polymerizability of rabbit skeletal tropomyosin: effects of enzymic and chemical modifications. Biochemistry. 1977 May 17;16(10):2264–2269. doi: 10.1021/bi00629a035. [DOI] [PubMed] [Google Scholar]
- Leavis P. C., Gergely J. Thin filament proteins and thin filament-linked regulation of vertebrate muscle contraction. CRC Crit Rev Biochem. 1984;16(3):235–305. doi: 10.3109/10409238409108717. [DOI] [PubMed] [Google Scholar]
- Lehrer S. S., Graceffa P., Betteridge D. Conformational dynamics of tropomyosin in solution: evidence for two conformational states. Ann N Y Acad Sci. 1981;366:285–299. doi: 10.1111/j.1749-6632.1981.tb20762.x. [DOI] [PubMed] [Google Scholar]
- Lehrer S. S. Intramolecular crosslinking of tropomyosin via disulfide bond formation: evidence for chain register. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3377–3381. doi: 10.1073/pnas.72.9.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer S. S., Morris E. P. Dual effects of tropomyosin and troponin-tropomyosin on actomyosin subfragment 1 ATPase. J Biol Chem. 1982 Jul 25;257(14):8073–8080. [PubMed] [Google Scholar]
- Lin T. I. Excimer fluorescence of pyrene-tropomyosin adducts. Biophys Chem. 1982 Jul;15(4):277–288. doi: 10.1016/0301-4622(82)80011-2. [DOI] [PubMed] [Google Scholar]
- Lu R. C., Lehrer S. S. Effects of interchain disulfide cross-links on the trypsin cleavage pattern and conformation of myosin subfragment 2. Biochemistry. 1984 Dec 4;23(25):5975–5981. doi: 10.1021/bi00320a013. [DOI] [PubMed] [Google Scholar]
- OOI T., MIHASHI K., KOBAYASHI H. On the polymerization of tropomyosin. Arch Biochem Biophys. 1962 Jul;98:1–11. doi: 10.1016/0003-9861(62)90138-8. [DOI] [PubMed] [Google Scholar]
- Pearlstone J. R., Smillie L. B. Binding of troponin-T fragments to several types of tropomyosin. Sensitivity to Ca2+ in the presence of troponin-C. J Biol Chem. 1982 Sep 25;257(18):10587–10592. [PubMed] [Google Scholar]
- Pearlstone J. R., Smillie L. B. Effects of troponin-I plus-C on the binding of troponin-T and its fragments to alpha-tropomyosin. Ca2+ sensitivity and cooperativity. J Biol Chem. 1983 Feb 25;258(4):2534–2542. [PubMed] [Google Scholar]
- Sato A., Mihashi K. Thermal modification of structure of tropomyosin. I. Changes in the intensity and polarization of the intrinsic fluorescence (tyrosine). J Biochem. 1972 Apr;71(4):597–605. [PubMed] [Google Scholar]
- Ueno H. Local structural changes in tropomyosin detected by a trypsin-probe method. Biochemistry. 1984 Sep 25;23(20):4791–4798. doi: 10.1021/bi00315a040. [DOI] [PubMed] [Google Scholar]
- Ueno H., Tawada Y., Ooi T. Properties of non-polymerizable tropomyosin obtained by carboxypeptidase A digestion. J Biochem. 1976 Aug;80(2):283–290. doi: 10.1093/oxfordjournals.jbchem.a131275. [DOI] [PubMed] [Google Scholar]
- Weber G. Uses of fluorescence in biophysics: some recent developments. Annu Rev Biophys Bioeng. 1972;1:553–570. doi: 10.1146/annurev.bb.01.060172.003005. [DOI] [PubMed] [Google Scholar]
- Woods E. F. The conformational stabilities of tropomyosins. Aust J Biol Sci. 1976 Dec;29(5-6):405–418. doi: 10.1071/bi9760405. [DOI] [PubMed] [Google Scholar]
- Wu C. W., Yarbrough L. R. N-(1-pyrene)maleimide: a fluorescent cross-linking reagent. Biochemistry. 1976 Jun 29;15(13):2863–2868. doi: 10.1021/bi00658a025. [DOI] [PubMed] [Google Scholar]


