Abstract
A model for the thermotropic transitions between lamellar (L alpha) and inverted hexagonal (HII) phases is developed. According to this model, the first structures to form during the L alpha----HII transition are inverted micellar intermediates (IMI). The structure, formation rates, and half-lives of IMI ("lipidic particles") were described previously. IMI coalesce in the planes between apposed bilayers to form two types of HII phase precursors. The first is a monolayer-encapsulated HII tube (RMI), which forms via coalescence of IMI in pearl-string fashion. These structures have been proposed previously based on electron microscopic evidence. I show that if only RMI form, L alpha in equilibrium HII transitions cannot occur on observed time scales (faster than seconds). I propose that a second type of intermediate, a line defect (LD), forms as well. LD should form via IMI-IMI coalescence in significant numbers, and elongate rapidly into structures consisting of two apposed halves of HII tubes. Transitions via LD can occur in less than seconds, the time depending on the fraction of IMI-IMI coalescence events producing LD and the number of IMI per unit of bilayer area. Hysteresis in the phase transition temperature may be due to the difference in water content of the two phases and their low water permeabilities. The model is in qualitative agreement with morphological, NMR, and x-ray diffraction data on phospholipid systems. The results are relevant to IMI-mediated interactions between unilamellar bilayer vesicles, and to the structure of inverted cubic phases observed in some phospholipid systems. These will be discussed in subsequent publications (D. P. Siegel, manuscript in preparation).
Full text
PDF
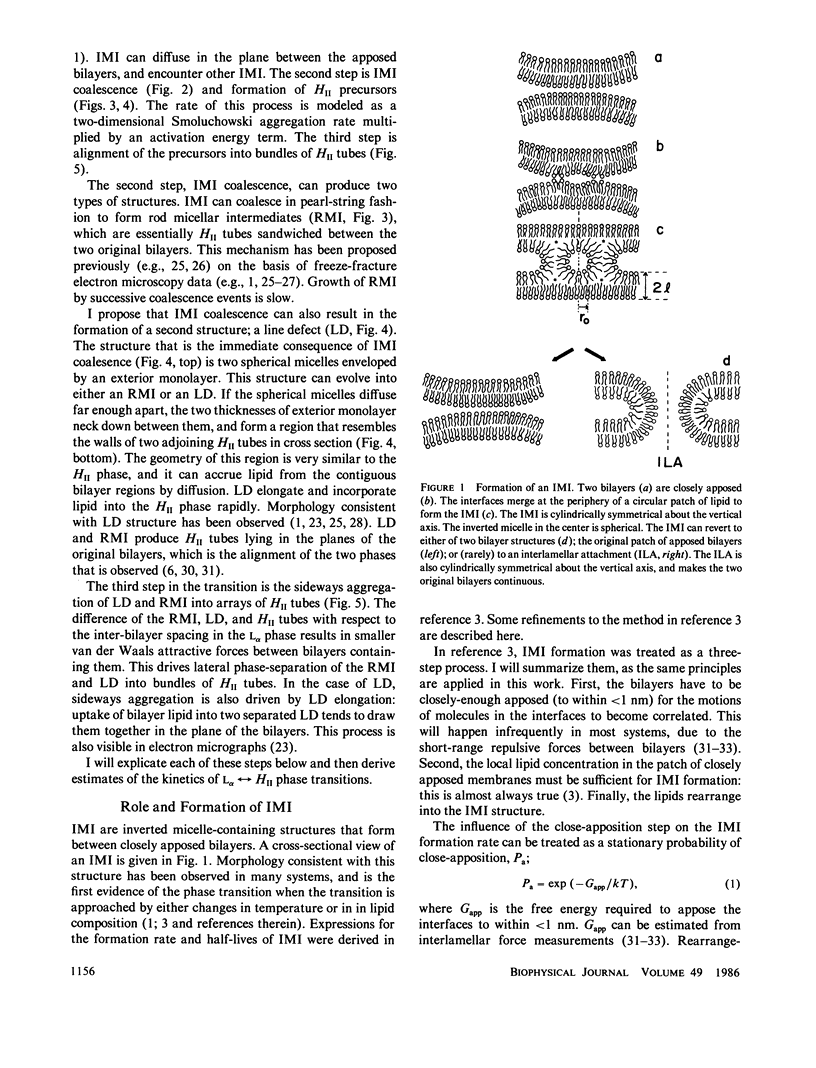


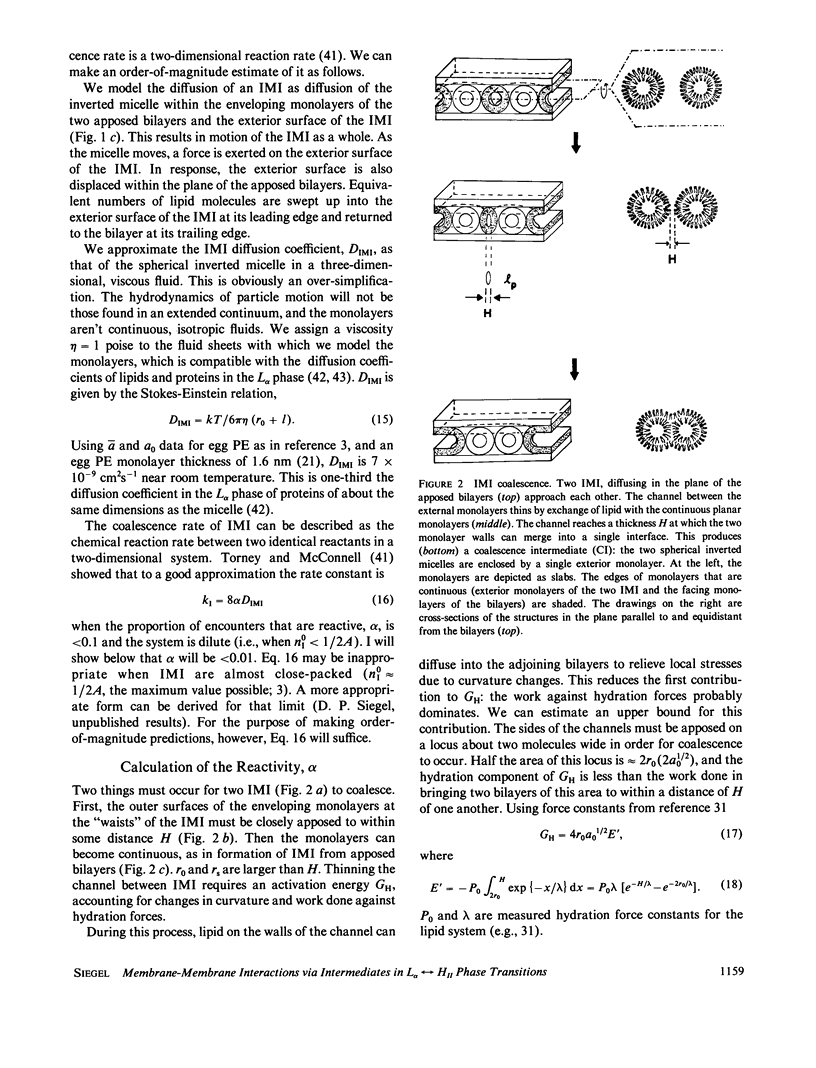
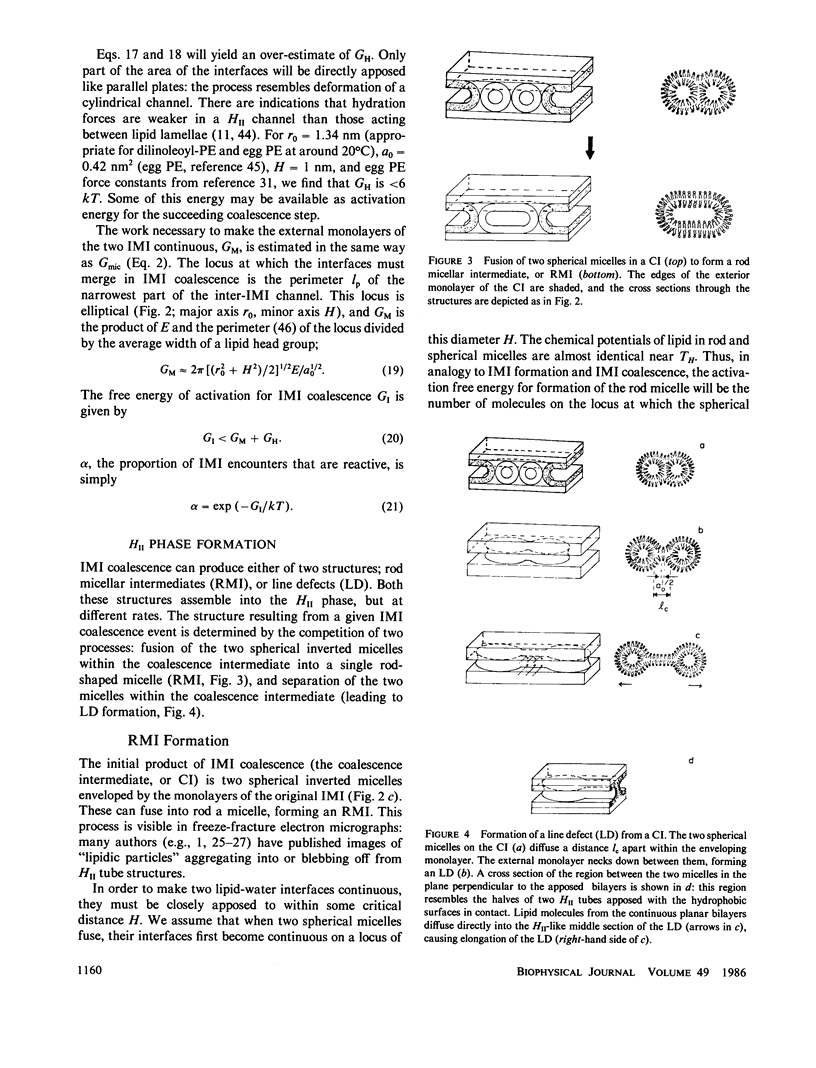
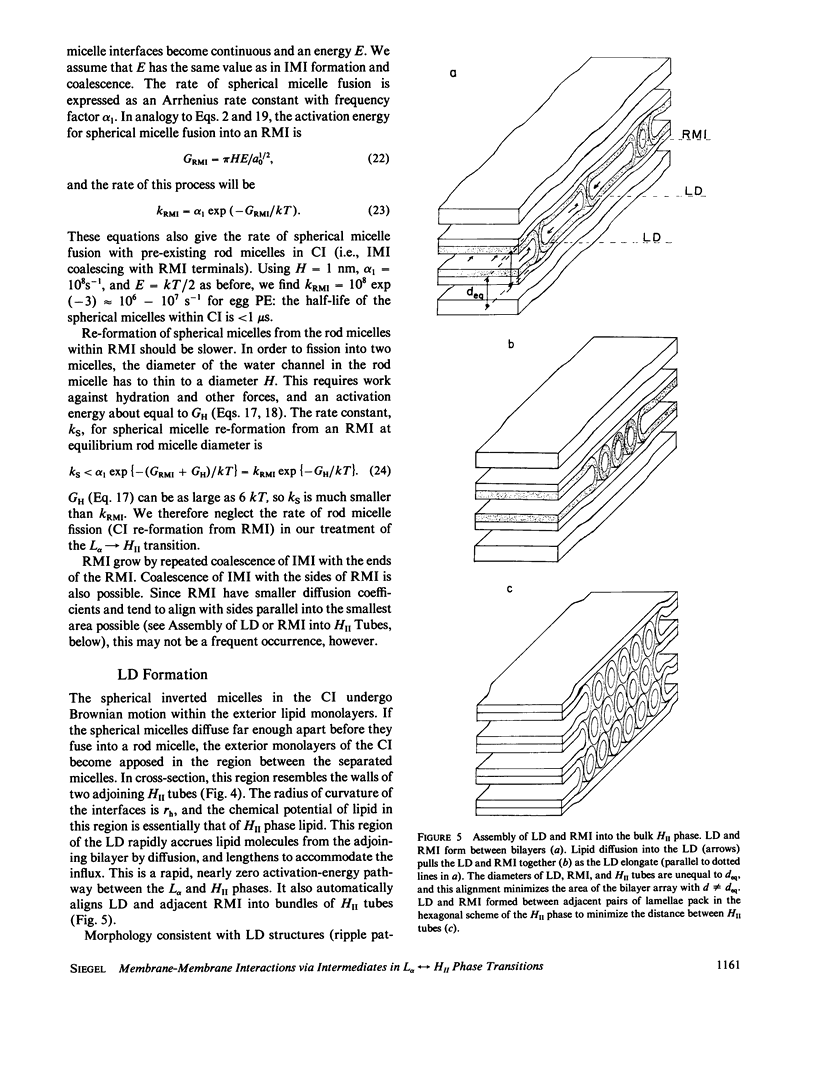









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert A. D., Sen A., Yeagle P. L. The effect of calcium on the bilayer stability of lipids from bovine rod outer segment disk membranes. Biochim Biophys Acta. 1984 Mar 28;771(1):28–34. doi: 10.1016/0005-2736(84)90106-8. [DOI] [PubMed] [Google Scholar]
- Bearer E. L., Düzgünes N., Friend D. S., Papahadjopoulos D. Fusion of phospholipid vesicles arrested by quick-freezing. The question of lipidic particles as intermediates in membrane fusion. Biochim Biophys Acta. 1982 Dec 8;693(1):93–98. doi: 10.1016/0005-2736(82)90474-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boni L. T., Hui S. W. Polymorphic phase behaviour of dilinoleoylphosphatidylethanolamine and palmitoyloleoylphosphatidylcholine mixtures. Structural changes between hexagonal, cubic and bilayer phases. Biochim Biophys Acta. 1983 Jun 10;731(2):177–185. doi: 10.1016/0005-2736(83)90007-x. [DOI] [PubMed] [Google Scholar]
- Borovjagin V. L., Vergara J. A., McIntosh T. J. Morphology of the intermediate stages in the lamellar to hexagonal lipid phase transition. J Membr Biol. 1982;69(3):199–212. doi: 10.1007/BF01870399. [DOI] [PubMed] [Google Scholar]
- Caffrey M. Kinetics and mechanism of the lamellar gel/lamellar liquid-crystal and lamellar/inverted hexagonal phase transition in phosphatidylethanolamine: a real-time X-ray diffraction study using synchrotron radiation. Biochemistry. 1985 Aug 27;24(18):4826–4844. doi: 10.1021/bi00339a017. [DOI] [PubMed] [Google Scholar]
- Cowley A. C., Fuller N. L., Rand R. P., Parsegian V. A. Measurement of repulsive forces between charged phospholipid bilayers. Biochemistry. 1978 Jul 25;17(15):3163–3168. doi: 10.1021/bi00608a034. [DOI] [PubMed] [Google Scholar]
- Crowe L. M., Crowe J. H. Hydration-dependent hexagonal phase lipid in a biological membrane. Arch Biochem Biophys. 1982 Sep;217(2):582–587. doi: 10.1016/0003-9861(82)90540-9. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., De Kruijff B. Polymorphic phase behaviour of lipid mixtures as detected by 31P NMR. Evidence that cholesterol may destabilize bilayer structure in membrane systems containing phosphatidylethanolamine. Biochim Biophys Acta. 1978 Feb 21;507(2):207–218. doi: 10.1016/0005-2736(78)90417-0. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., de Kruijff B., Hope M. J., Nayar R., Schmid S. L. Phospholipids and membrane transport. Can J Biochem. 1980 Oct;58(10):1091–1100. doi: 10.1139/o80-147. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., van Dijck P. W., de Kruijff B., de Gier J. Effects of cholesterol on the properties of equimolar mixtures of synthetic phosphatidylethanolamine and phosphatidylcholine. A 31P NMR and differential scanning calorimetry study. Biochim Biophys Acta. 1978 Oct 19;513(1):21–30. doi: 10.1016/0005-2736(78)90108-6. [DOI] [PubMed] [Google Scholar]
- De Kruijff B., Verkleij A. J., Leunissen-Bijvelt J., Van Echteld C. J., Hille J., Rijnbout H. Further aspects of the Ca2+-dependent polymorphism of bovine heart cardiolipin. Biochim Biophys Acta. 1982 Dec 8;693(1):1–12. doi: 10.1016/0005-2736(82)90464-3. [DOI] [PubMed] [Google Scholar]
- Düzgüneş N., Wilschut J., Fraley R., Papahadjopoulos D. Studies on the mechanism of membrane fusion. Role of head-group composition in calcium- and magnesium-induced fusion of mixed phospholipid vesicles. Biochim Biophys Acta. 1981 Mar 20;642(1):182–195. doi: 10.1016/0005-2736(81)90148-6. [DOI] [PubMed] [Google Scholar]
- Ellens H., Bentz J., Szoka F. C. pH-induced destabilization of phosphatidylethanolamine-containing liposomes: role of bilayer contact. Biochemistry. 1984 Mar 27;23(7):1532–1538. doi: 10.1021/bi00302a029. [DOI] [PubMed] [Google Scholar]
- Galla H. J., Hartmann W., Theilen U., Sackmann E. On two-dimensional passive random walk in lipid bilayers and fluid pathways in biomembranes. J Membr Biol. 1979 Jul 31;48(3):215–236. doi: 10.1007/BF01872892. [DOI] [PubMed] [Google Scholar]
- Gamble R. C., Schimmel P. R. Nanosecond relaxation processes of phospholipid bilayers in the transition zone. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3011–3014. doi: 10.1073/pnas.75.7.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruner S. M., Rothschild K. J., Clark N. A. X-ray diffraction and electron microscope study of phase separation in rod outer segment photoreceptor membrane multilayers. Biophys J. 1982 Sep;39(3):241–251. doi: 10.1016/S0006-3495(82)84514-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hah J. S., Hui S. W., Jung C. Y. Effects of physical states of phospholipids on the incorporation and cytochalasin B binding activity of human erythrocyte membrane proteins in reconstituted vesicles. Biochemistry. 1983 Sep 27;22(20):4763–4769. doi: 10.1021/bi00289a023. [DOI] [PubMed] [Google Scholar]
- Hardman P. D. Spin-label characterisation of the lamellar-to-hexagonal (HII) phase transition in egg phosphatidylethanolamine aqueous dispersions. Eur J Biochem. 1982 May;124(1):95–101. doi: 10.1111/j.1432-1033.1982.tb05910.x. [DOI] [PubMed] [Google Scholar]
- Helfrich W. Elastic properties of lipid bilayers: theory and possible experiments. Z Naturforsch C. 1973 Nov-Dec;28(11):693–703. doi: 10.1515/znc-1973-11-1209. [DOI] [PubMed] [Google Scholar]
- Hope M. J., Cullis P. R. The bilayer stability of inner monolayer lipids from the human erythrocyte. FEBS Lett. 1979 Nov 15;107(2):323–326. doi: 10.1016/0014-5793(79)80399-3. [DOI] [PubMed] [Google Scholar]
- Hui S. W., Stewart T. P. 'Lipidic particles' are intermembrane attachment sites. Nature. 1981 Apr 2;290(5805):427–428. doi: 10.1038/290427a0. [DOI] [PubMed] [Google Scholar]
- Hui S. W., Stewart T. P., Boni L. T. The nature of lipidic particles and their roles in polymorphic transitions. Chem Phys Lipids. 1983 Aug;33(2):113–126. doi: 10.1016/0009-3084(83)90015-4. [DOI] [PubMed] [Google Scholar]
- Hui S. W., Stewart T. P., Yeagle P. L., Albert A. D. Bilayer to non-bilayer transition in mixtures of phosphatidylethanolamine and phosphatidylcholine: implications for membrane properties. Arch Biochem Biophys. 1981 Apr 1;207(2):227–240. doi: 10.1016/0003-9861(81)90029-1. [DOI] [PubMed] [Google Scholar]
- Kolber M. A., Haynes D. H. Evidence for a role of phosphatidyl ethanolamine as a modulator of membrane-membrane contact. J Membr Biol. 1979 Jun 29;48(1):95–114. doi: 10.1007/BF01869258. [DOI] [PubMed] [Google Scholar]
- Lau A. L., Chan S. I. Alamethicin-mediated fusion of lecithin vesicles. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2170–2174. doi: 10.1073/pnas.72.6.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis L. J., McAlister M., Fuller N., Rand R. P., Parsegian V. A. Measurement of the lateral compressibility of several phospholipid bilayers. Biophys J. 1982 Mar;37(3):667–672. [PMC free article] [PubMed] [Google Scholar]
- Nayar R., Hope M. J., Cullis P. R. Phospholipids as adjuncts for calcium ion stimulated release of chromaffin granule contents: implications for mechanisms of exocytosis. Biochemistry. 1982 Sep 14;21(19):4583–4589. doi: 10.1021/bi00262a011. [DOI] [PubMed] [Google Scholar]
- Peters R., Cherry R. J. Lateral and rotational diffusion of bacteriorhodopsin in lipid bilayers: experimental test of the Saffman-Delbrück equations. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4317–4321. doi: 10.1073/pnas.79.14.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranck J. L., Letellier L., Shechter E., Krop B., Pernot P., Tardieu A. X-ray analysis of the kinetics of Escherichia coli lipid and membrane structural transitions. Biochemistry. 1984 Oct 9;23(21):4955–4961. doi: 10.1021/bi00316a020. [DOI] [PubMed] [Google Scholar]
- Rand R. P. Interacting phospholipid bilayers: measured forces and induced structural changes. Annu Rev Biophys Bioeng. 1981;10:277–314. doi: 10.1146/annurev.bb.10.060181.001425. [DOI] [PubMed] [Google Scholar]
- Rand R. P., Sengupta S. Cardiolipin forms hexagonal structures with divalent cations. Biochim Biophys Acta. 1972 Feb 11;255(2):484–492. doi: 10.1016/0005-2736(72)90152-6. [DOI] [PubMed] [Google Scholar]
- Reiss-Husson F. Structure des phases liquide-cristallines de différents phospholipides, monoglycérides, sphingolipides, anhydres ou en présence d'eau. J Mol Biol. 1967 May 14;25(3):363–382. doi: 10.1016/0022-2836(67)90192-1. [DOI] [PubMed] [Google Scholar]
- Sakurai I., Kawamura Y. Magnetic-field-induced orientation and bending of the myelin figures of phosphatidylcholine. Biochim Biophys Acta. 1983 Oct 26;735(1):189–192. doi: 10.1016/0005-2736(83)90275-4. [DOI] [PubMed] [Google Scholar]
- Servuss R. M., Harbich W., Helfrich W. Measurement of the curvature-elastic modulus of egg lecithin bilayers. Biochim Biophys Acta. 1976 Jul 15;436(4):900–903. doi: 10.1016/0005-2736(76)90422-3. [DOI] [PubMed] [Google Scholar]
- Siegel D. P. Inverted micellar structures in bilayer membranes. Formation rates and half-lives. Biophys J. 1984 Feb;45(2):399–420. doi: 10.1016/S0006-3495(84)84164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundler R., Düzgüneş N., Papahadjopoulos D. Control of membrane fusion by phospholipid head groups. II. The role of phosphatidylethanolamine in mixtures with phosphatidate and phosphatidylinositol. Biochim Biophys Acta. 1981 Dec 21;649(3):751–758. doi: 10.1016/0005-2736(81)90180-2. [DOI] [PubMed] [Google Scholar]
- Taraschi T. F., van der Steen A. T., de Kruijff B., Tellier C., Verkleij A. J. Lectin-receptor interactions in liposomes: evidence that binding of wheat germ agglutinin to glycoprotein-phosphatidylethanolamine vesicles induces nonbilayer structures. Biochemistry. 1982 Nov 9;21(23):5756–5764. doi: 10.1021/bi00266a005. [DOI] [PubMed] [Google Scholar]
- Tilcock C. P., Bally M. B., Farren S. B., Cullis P. R. Influence of cholesterol on the structural preferences of dioleoylphosphatidylethanolamine-dioleoylphosphatidylcholine systems: a phosphorus-31 and deuterium nuclear magnetic resonance study. Biochemistry. 1982 Sep 14;21(19):4596–4601. doi: 10.1021/bi00262a013. [DOI] [PubMed] [Google Scholar]
- Van Venetie R., Verkleij A. J. Analysis of the hexagonal II phase and its relations to lipidic particles and the lamellar phase. A freeze-fracture study. Biochim Biophys Acta. 1981 Jul 20;645(2):262–269. doi: 10.1016/0005-2736(81)90197-8. [DOI] [PubMed] [Google Scholar]
- Van Venetië R., Verkleij A. J. Possible role of non-bilayer lipids in the structure of mitochondria. A freeze-fracture electron microscopy study. Biochim Biophys Acta. 1982 Nov 22;692(3):397–405. doi: 10.1016/0005-2736(82)90390-x. [DOI] [PubMed] [Google Scholar]
- Vaz W. L., Criado M., Madeira V. M., Schoellmann G., Jovin T. M. Size dependence of the translational diffusion of large integral membrane proteins in liquid-crystalline phase lipid bilayers. A study using fluorescence recovery after photobleaching. Biochemistry. 1982 Oct 26;21(22):5608–5612. doi: 10.1021/bi00265a034. [DOI] [PubMed] [Google Scholar]
- Verkleij A. J. Lipidic intramembranous particles. Biochim Biophys Acta. 1984 Jan 27;779(1):43–63. doi: 10.1016/0304-4157(84)90003-0. [DOI] [PubMed] [Google Scholar]
- Verkleij A. J., van Echteld C. J., Gerritsen W. J., Cullis P. R., de Kruijff B. The lipidic particle as an intermediate structure in membrane fusion processes and bilayer to hexagonal HII transitions. Biochim Biophys Acta. 1980 Aug 14;600(3):620–624. doi: 10.1016/0005-2736(80)90465-4. [DOI] [PubMed] [Google Scholar]
- Wilschut J., Holsappel M., Jansen R. Ca2+-induced fusion of cardiolipin/phosphatidylcholine vesicles monitored by mixing of aqueous contents. Biochim Biophys Acta. 1982 Sep 9;690(2):297–301. doi: 10.1016/0005-2736(82)90334-0. [DOI] [PubMed] [Google Scholar]
- de Kruijff B., Cullis P. R. Cytochrome c specifically induces non-bilayer structures in cardiolipin-containing model membranes. Biochim Biophys Acta. 1980 Nov 18;602(3):477–490. doi: 10.1016/0005-2736(80)90327-2. [DOI] [PubMed] [Google Scholar]
- van Duijn G., Verkleij A. J., de Kruijff B. Influence of phospholipid peroxidation on the phase behavior of phosphatidylcholine and phosphatidylethanolamine in aqueous dispersions. Biochemistry. 1984 Oct 9;23(21):4969–4977. doi: 10.1021/bi00316a022. [DOI] [PubMed] [Google Scholar]


