Abstract
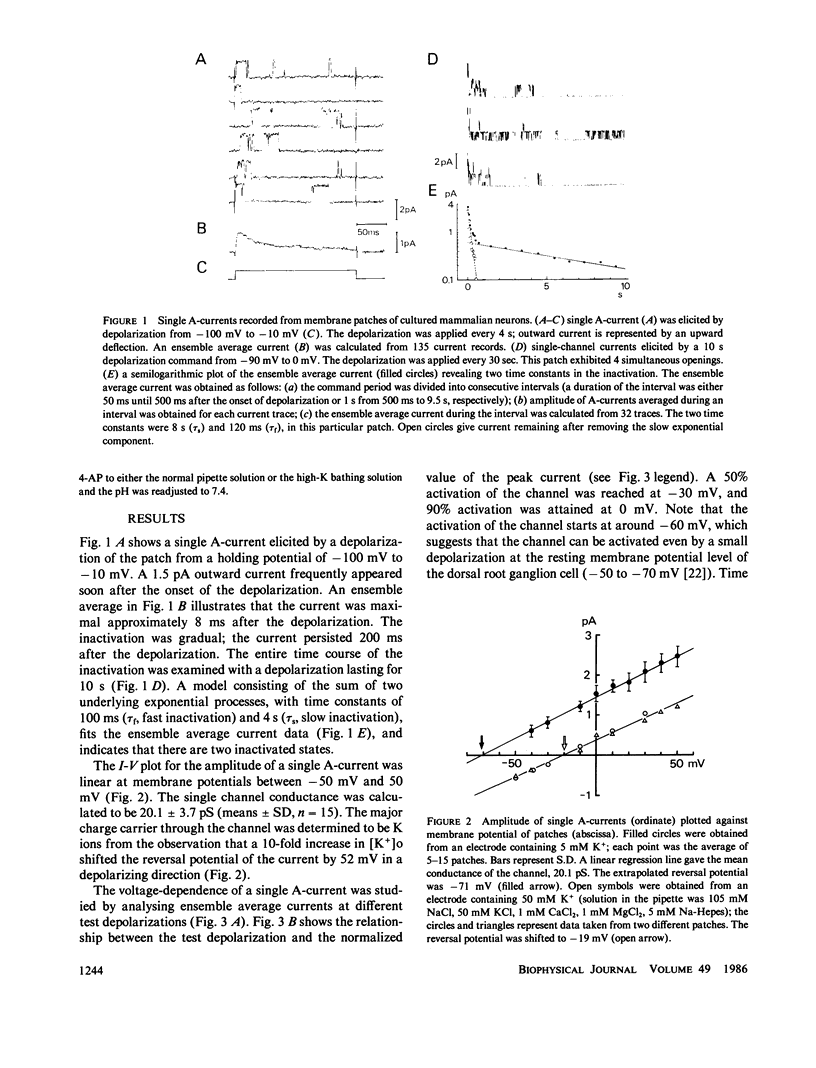
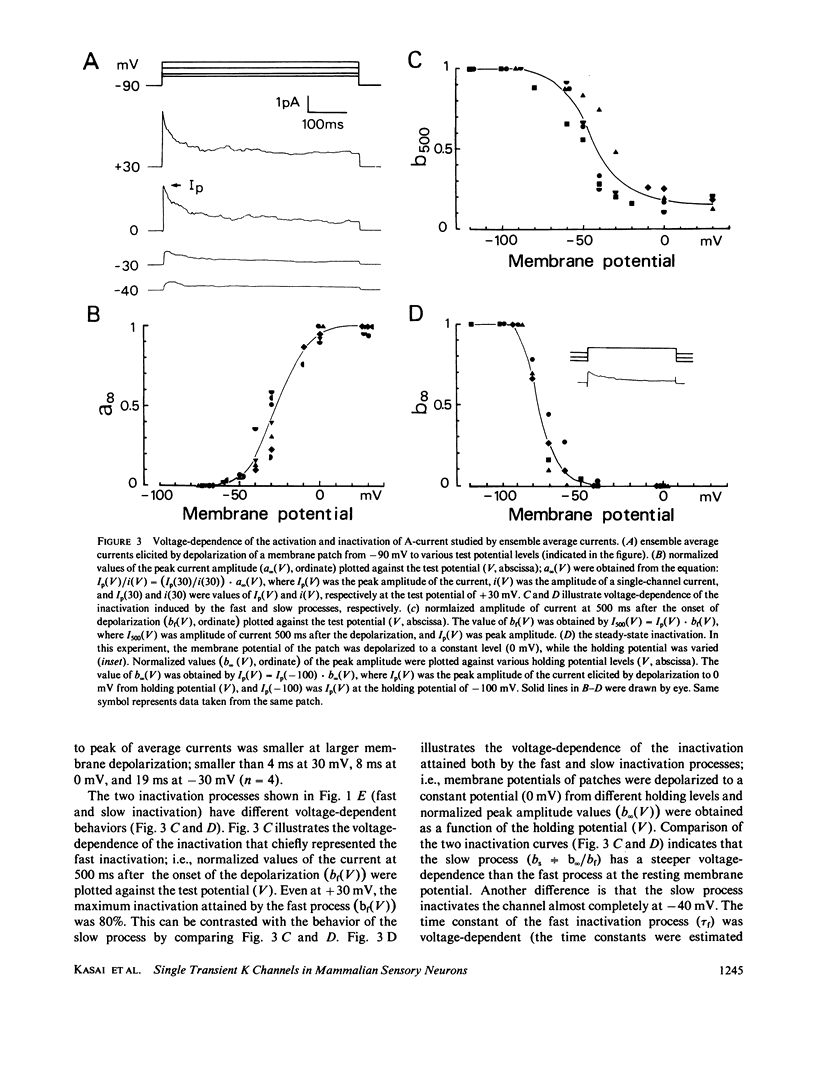
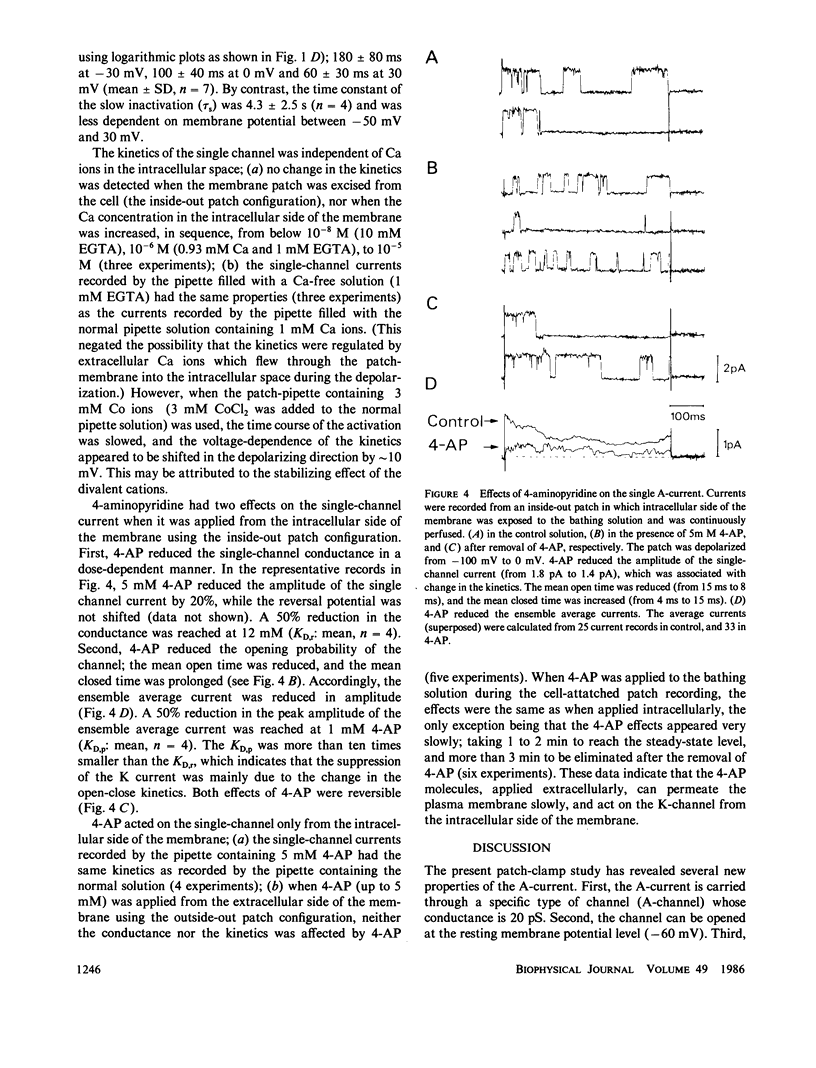
A single-channel recording of the transient outward current (A-current) was obtained from dorsal root ganglion cells in culture using patch-clamp techniques. Depolarization of the membrane patch elicited pulse like current of a uniform amplitude in an outward direction, of which the unitary conductance was 20 pS. Alteration of extracellular ionic compositions indicated that the charge carriers were K ions. A systematic study was made on the voltage-dependence of the ensemble average current; (a) the activation started at a potential around -60 mV; (b) the time course of the activation was relatively rapid; (c) the channel was completely inactivated at a potential positive to -40 mV. Two time constants (tau f = 100 ms and tau s = 4,000 ms) were detected in the decay of the current indicating that the channels had two different states of inactivation. A convulsant, 4-aminopyridine (4-AP), acted on the channel only from the intracellular side of the membrane. 4-AP (5 mM) reduced not only mean open time (by 50%) but also the single-channel conductance (by 20%). The properties of the channel were independent of Ca ions in the intracellular space.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byrne J. H., Shapiro E., Dieringer N., Koester J. Biophysical mechanisms contributing to inking behavior in Aplysia. J Neurophysiol. 1979 Sep;42(5):1233–1250. doi: 10.1152/jn.1979.42.5.1233. [DOI] [PubMed] [Google Scholar]
- Connor J. A. Neural repetitive firing: a comparative study of membrane properties of crustacean walking leg axons. J Neurophysiol. 1975 Jul;38(4):922–932. doi: 10.1152/jn.1975.38.4.922. [DOI] [PubMed] [Google Scholar]
- Connor J. A. Slow repetitive activity from fast conductance changes in neurons. Fed Proc. 1978 Jun;37(8):2139–2145. [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Prediction of repetitive firing behaviour from voltage clamp data on an isolated neurone soma. J Physiol. 1971 Feb;213(1):31–53. doi: 10.1113/jphysiol.1971.sp009366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F., Neher E. Single channel recordings of K+ currents in squid axons. Nature. 1980 May 15;285(5761):140–143. doi: 10.1038/285140a0. [DOI] [PubMed] [Google Scholar]
- Daut J. Modulation of the excitatory synaptic response by fast transient K+ current in snail neurones. Nat New Biol. 1973 Dec 19;246(155):193–196. doi: 10.1038/newbio246193a0. [DOI] [PubMed] [Google Scholar]
- Dekin M. S., Getting P. A. Firing pattern of neurons in the nucleus tractus solitarius: modulation by membrane hyperpolarization. Brain Res. 1984 Dec 17;324(1):180–184. doi: 10.1016/0006-8993(84)90640-1. [DOI] [PubMed] [Google Scholar]
- Dubois J. M. Evidence for the existence of three types of potassium channels in the frog Ranvier node membrane. J Physiol. 1981 Sep;318:297–316. doi: 10.1113/jphysiol.1981.sp013865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda J., Kameyama M. Enhancement of Ca spikes in nerve cells of adult mammals during neurite growth in tissue culture. Nature. 1979 Jun 7;279(5713):546–548. doi: 10.1038/279546a0. [DOI] [PubMed] [Google Scholar]
- Galvan M., Sedlmeir C. Outward currents in voltage-clamped rat sympathetic neurones. J Physiol. 1984 Nov;356:115–133. doi: 10.1113/jphysiol.1984.sp015456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B., Galvan M., Grafe P., Wigström H. A transient outward current in a mammalian central neurone blocked by 4-aminopyridine. Nature. 1982 Sep 16;299(5880):252–254. doi: 10.1038/299252a0. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Puil E., Werman R. EGTA and motoneuronal after-potentials. J Physiol. 1978 Feb;275:199–223. doi: 10.1113/jphysiol.1978.sp012186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol. 1980 Aug;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature. 1981 Jun 11;291(5815):497–500. doi: 10.1038/291497a0. [DOI] [PubMed] [Google Scholar]
- Neher E. Two fast transient current components during voltage clamp on snail neurons. J Gen Physiol. 1971 Jul;58(1):36–53. doi: 10.1085/jgp.58.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATO M., AUSTIN G. Intracellular potentials of mammalian dorsal root ganglion cells. J Neurophysiol. 1961 Nov;24:569–582. doi: 10.1152/jn.1961.24.6.569. [DOI] [PubMed] [Google Scholar]
- Salkoff L., Wyman R. Facilitation of membrane electrical excitability in Drosophila. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6216–6220. doi: 10.1073/pnas.77.10.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. H. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977 Feb;265(2):465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]


