Abstract
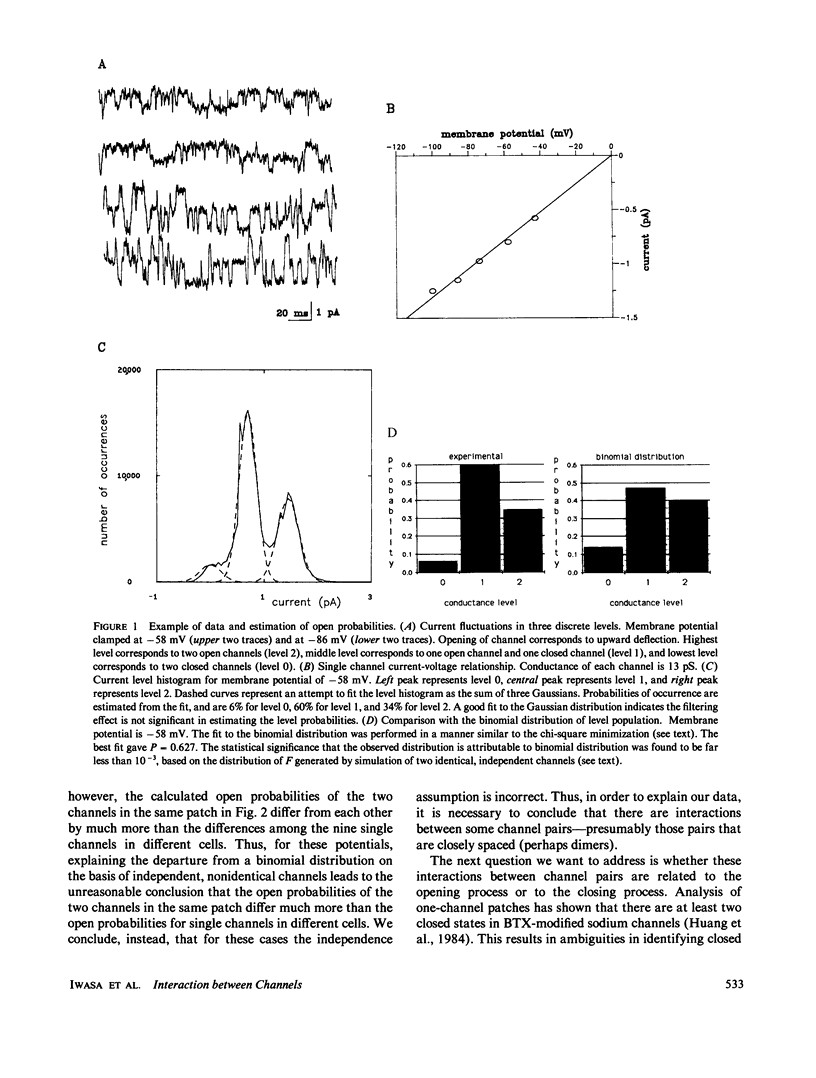
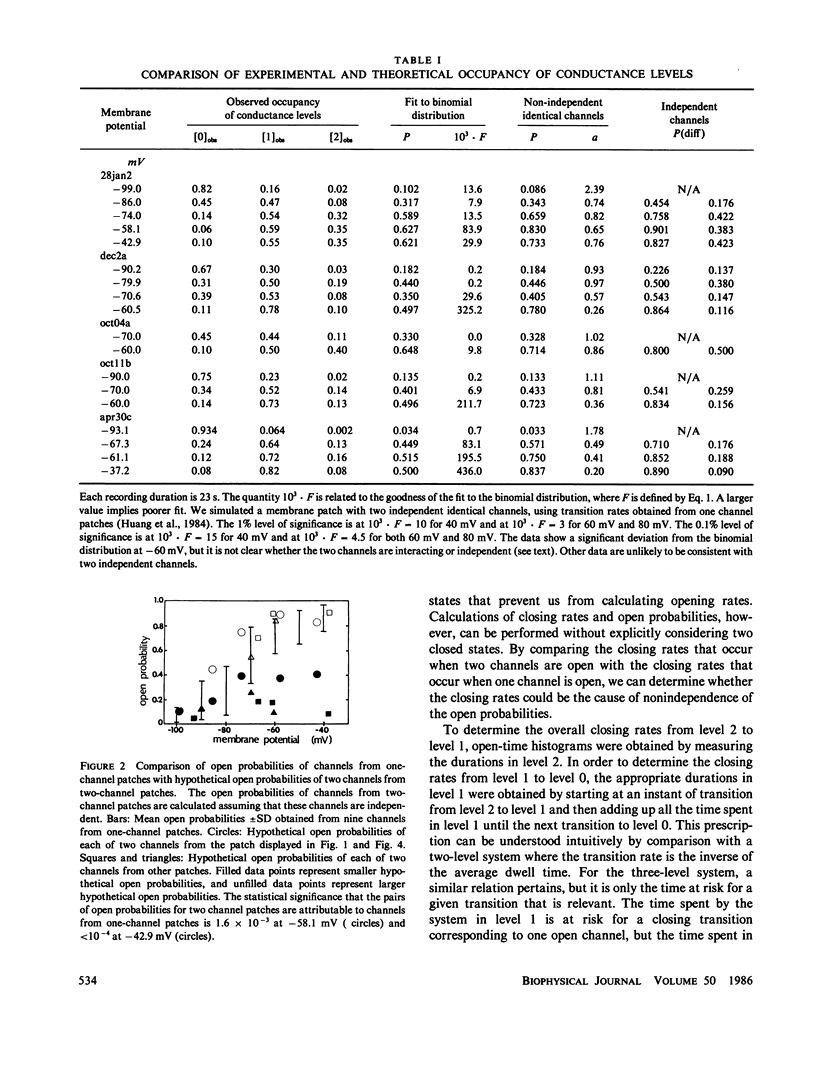
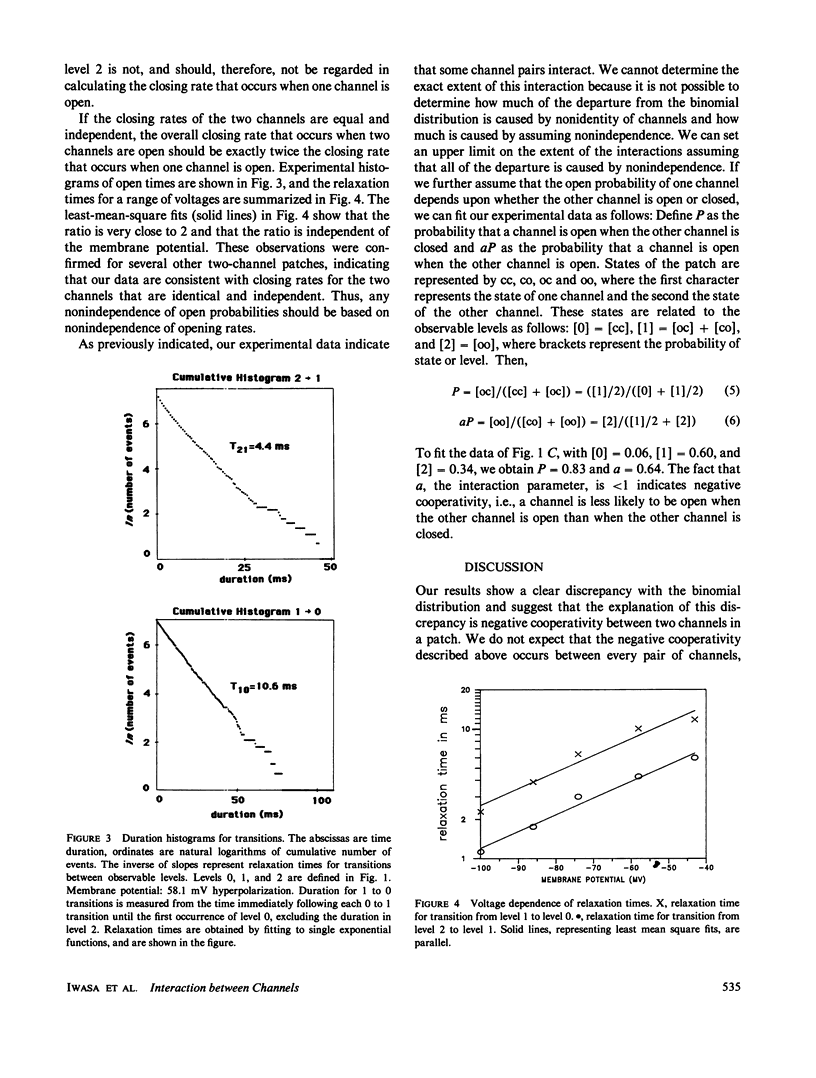
Current records from voltage-clamped membrane patches containing two batrachotoxin-modified sodium channels were analyzed to determine whether these channels are identical and independent. In most two-channel patches, the experimentally observed probabilities that zero, one, or two channels are open differ from the binomial distribution, demonstrating that the two channels are nonidentical or nonindependent or both. From the same current records, we also determined the rate for the transition from two open channels to one open channel and for the transition from one open channel to zero open channels. These data are consistent with closing rates for the two channels that are equal and independent. Both probability and closing rate data can be fit by a model wherein the channels are identical, the closing rates are independent, and the opening rate is greater when the other channel is closed than when it is open. The implications of this model for analyzing noise spectra and current variance are examined.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich R. W., Corey D. P., Stevens C. F. A reinterpretation of mammalian sodium channel gating based on single channel recording. Nature. 1983 Dec 1;306(5942):436–441. doi: 10.1038/306436a0. [DOI] [PubMed] [Google Scholar]
- Almers W., Stanfield P. R., Stühmer W. Lateral distribution of sodium and potassium channels in frog skeletal muscle: measurements with a patch-clamp technique. J Physiol. 1983 Mar;336:261–284. doi: 10.1113/jphysiol.1983.sp014580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W., Stirling C. Distribution of transport proteins over animal cell membranes. J Membr Biol. 1984;77(3):169–186. doi: 10.1007/BF01870567. [DOI] [PubMed] [Google Scholar]
- Angelides K. J., Nutter T. J. Molecular and cellular mapping of the voltage-dependent na channel. Biophys J. 1984 Jan;45(1):31–34. doi: 10.1016/S0006-3495(84)84096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A., Morrow C. S. Binding to saxitoxin to electrically excitable neuroblastoma cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):218–222. doi: 10.1073/pnas.75.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenstein G., Lecar H., Nossal R. The nature of the negative resistance in bimolecular lipid membranes containing excitability-inducing material. J Gen Physiol. 1970 Jan;55(1):119–133. doi: 10.1085/jgp.55.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Huang L. Y., Moran N., Ehrenstein G. Batrachotoxin modifies the gating kinetics of sodium channels in internally perfused neuroblastoma cells. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2082–2085. doi: 10.1073/pnas.79.6.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L. Y., Moran N., Ehrenstein G. Gating kinetics of batrachotoxin-modified sodium channels in neuroblastoma cells determined from single-channel measurements. Biophys J. 1984 Jan;45(1):313–322. doi: 10.1016/S0006-3495(84)84157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Stevens C. F. Conductance fluctuations and ionic pores in membranes. Annu Rev Biophys Bioeng. 1977;6:345–381. doi: 10.1146/annurev.bb.06.060177.002021. [DOI] [PubMed] [Google Scholar]
- Neumcke B., Stämpfli R. Alteration of the conductance of Na+ channels in the nodal membrane of frog nerve by holding potential and tetrodotoxin. Biochim Biophys Acta. 1983 Jan 5;727(1):177–184. doi: 10.1016/0005-2736(83)90382-6. [DOI] [PubMed] [Google Scholar]
- Sachs F., Neil J., Barkakati N. The automated analysis of data from single ionic channels. Pflugers Arch. 1982 Dec;395(4):331–340. doi: 10.1007/BF00580798. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J. The variance of sodium current fluctuations at the node of Ranvier. J Physiol. 1980 Oct;307:97–129. doi: 10.1113/jphysiol.1980.sp013426. [DOI] [PMC free article] [PubMed] [Google Scholar]


