Abstract
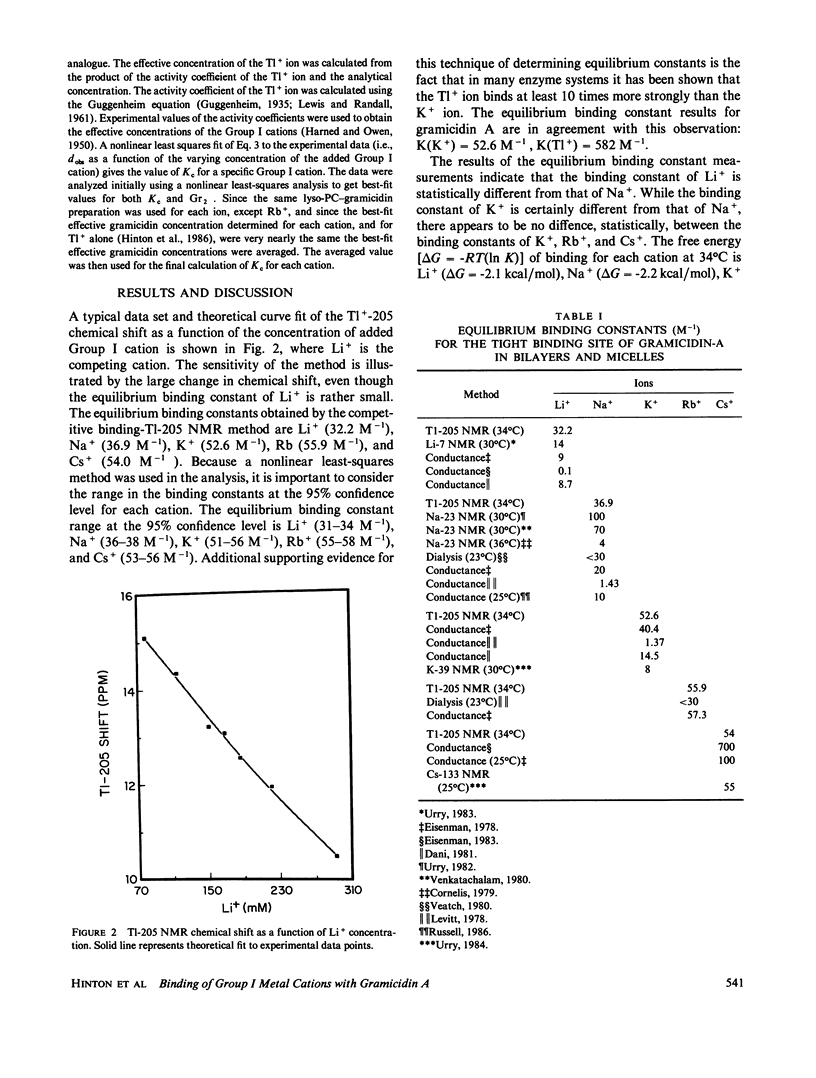
The equilibrium binding constants of the Group I metal cations with gramicidin A in aqueous dispersions of lyso-PC have been determined using a combination of competitive binding with the T1+ ion and T1-205 NMR spectroscopy. The values of the binding constants at 34 degrees C are Li (32.2 M-1), Na (36.9 M-1), K (52.6 M-1), Rb (55.9 M-1), and Cs (54.0 M-1). The equilibrium binding constant for the T1+ ion at this temperature is 582 M-1. The relationships between the binding constants, the free energy of the binding process, and the cation selectivity of the gramicidin A channel are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen O. S. Ion movement through gramicidin A channels. Studies on the diffusion-controlled association step. Biophys J. 1983 Feb;41(2):147–165. doi: 10.1016/S0006-3495(83)84416-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornélis A., Laszlo P. Sodium binding sites of gramicidin A: sodium-23 nuclear magnetic resonance study. Biochemistry. 1979 May 15;18(10):2004–2007. doi: 10.1021/bi00577a025. [DOI] [PubMed] [Google Scholar]
- Dani J. A., Levitt D. G. Binding constants of Li+, K+, and Tl+ in the gramicidin channel determined from water permeability measurements. Biophys J. 1981 Aug;35(2):485–499. doi: 10.1016/S0006-3495(81)84804-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman G., Horn R. Ionic selectivity revisited: the role of kinetic and equilibrium processes in ion permeation through channels. J Membr Biol. 1983;76(3):197–225. doi: 10.1007/BF01870364. [DOI] [PubMed] [Google Scholar]
- Eisenman G., Sandblom J. P. Modeling the gramicidin channel: interpretation of experimental data using rate theory. Biophys J. 1984 Jan;45(1):88–90. doi: 10.1016/S0006-3495(84)84119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman G., Sandblom J., Neher E. Interactions in cation permeation through the gramicidin channel. Cs, Rb, K, Na, Li, Tl, H, and effects of anion binding. Biophys J. 1978 May;22(2):307–340. doi: 10.1016/S0006-3495(78)85491-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
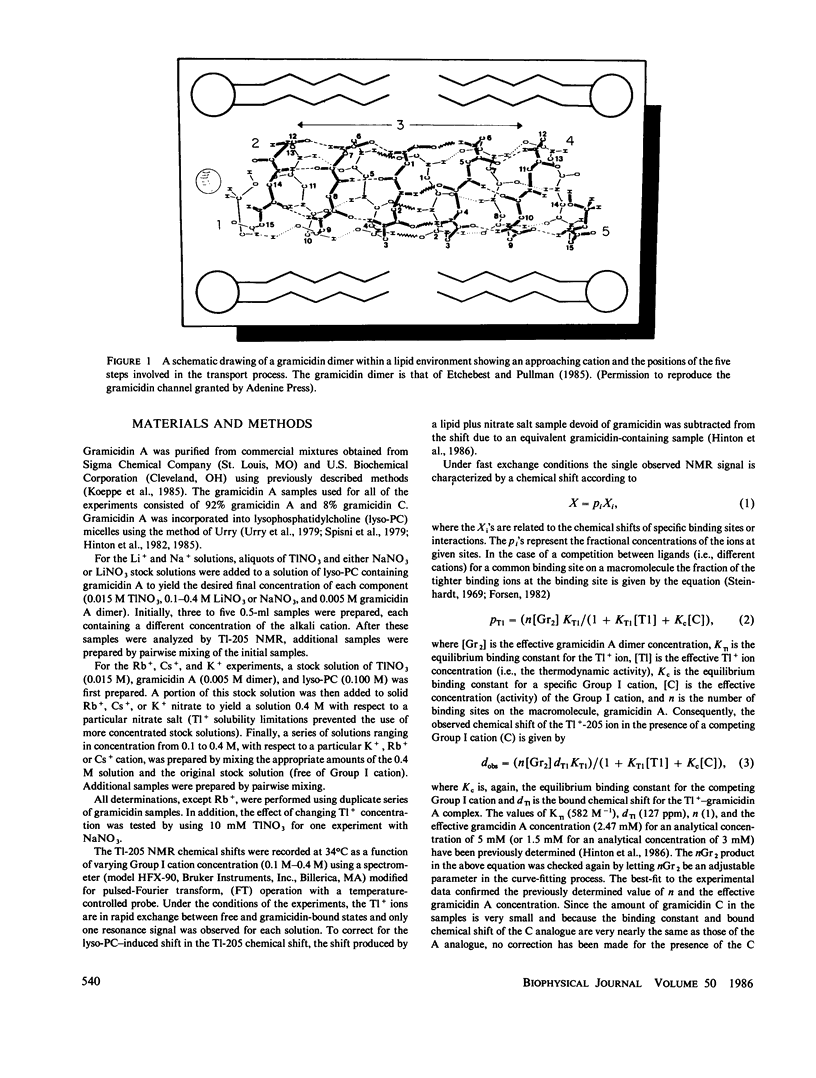
- Etchebest C., Pullman A. The effect of the amino-acid side chains on the energy profiles for ion transport in the gramicidin A channel. J Biomol Struct Dyn. 1985 Feb;2(5):859–870. doi: 10.1080/07391102.1985.10507605. [DOI] [PubMed] [Google Scholar]
- Finkelstein A., Andersen O. S. The gramicidin A channel: a review of its permeability characteristics with special reference to the single-file aspect of transport. J Membr Biol. 1981 Apr 30;59(3):155–171. doi: 10.1007/BF01875422. [DOI] [PubMed] [Google Scholar]
- Forsén S., Lindman B. Ion binding in biological systems as studied by NMR spectroscopy. Methods Biochem Anal. 1981;27:289–486. doi: 10.1002/9780470110478.ch5. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic selectivity, saturation, and block in sodium channels. A four-barrier model. J Gen Physiol. 1975 Nov;66(5):535–560. doi: 10.1085/jgp.66.5.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton J. F., Koeppe R. E., 2nd, Shungu D., Whaley W. L., Paczkowski J. A., Millett F. S. Equilibrium binding constants for Tl+ with gramicidins A, B and C in a lysophosphatidylcholine environment determined by 205Tl nuclear magnetic resonance spectroscopy. Biophys J. 1986 Feb;49(2):571–577. doi: 10.1016/S0006-3495(86)83668-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton J. F., Young G., Millett F. S. Thallous ion interaction with gramicidin incorporated in micelles studied by thallium-205 nuclear magnetic resonance. Biochemistry. 1982 Feb 16;21(4):651–654. doi: 10.1021/bi00533a009. [DOI] [PubMed] [Google Scholar]
- Hladky S. B., Haydon D. A. Ion transfer across lipid membranes in the presence of gramicidin A. I. Studies of the unit conductance channel. Biochim Biophys Acta. 1972 Aug 9;274(2):294–312. doi: 10.1016/0005-2736(72)90178-2. [DOI] [PubMed] [Google Scholar]
- Koeppe R. E., 2nd, Paczkowski J. A., Whaley W. L. Gramicidin K, a new linear channel-forming gramicidin from Bacillus brevis. Biochemistry. 1985 Jun 4;24(12):2822–2826. doi: 10.1021/bi00333a002. [DOI] [PubMed] [Google Scholar]
- Levitt D. G. Electrostatic calculations for an ion channel. II. Kinetic behavior of the gramicidin A channel. Biophys J. 1978 May;22(2):221–248. doi: 10.1016/S0006-3495(78)85486-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt D. G., Elias S. R., Hautman J. M. Number of water molecules coupled to the transport of sodium, potassium and hydrogen ions via gramicidin, nonactin or valinomycin. Biochim Biophys Acta. 1978 Sep 22;512(2):436–451. doi: 10.1016/0005-2736(78)90266-3. [DOI] [PubMed] [Google Scholar]
- Myers V. B., Haydon D. A. Ion transfer across lipid membranes in the presence of gramicidin A. II. The ion selectivity. Biochim Biophys Acta. 1972 Aug 9;274(2):313–322. doi: 10.1016/0005-2736(72)90179-4. [DOI] [PubMed] [Google Scholar]
- Russell E. W., Weiss L. B., Navetta F. I., Koeppe R. E., 2nd, Andersen O. S. Single-channel studies on linear gramicidins with altered amino acid side chains. Effects of altering the polarity of the side chain at position 1 in gramicidin A. Biophys J. 1986 Mar;49(3):673–686. doi: 10.1016/S0006-3495(86)83694-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spisni A., Khaled M. A., Urry D. W. Temperature-induced incorporation of gramicidin A into lysolecithin micelles demonstrated by 13C NMR. FEBS Lett. 1979 Jun 15;102(2):321–324. doi: 10.1016/0014-5793(79)80027-7. [DOI] [PubMed] [Google Scholar]
- Urban B. W., Hladky S. B., Haydon D. A. Ion movements in gramicidin pores. An example of single-file transport. Biochim Biophys Acta. 1980 Nov 4;602(2):331–354. doi: 10.1016/0005-2736(80)90316-8. [DOI] [PubMed] [Google Scholar]
- Urry D. W. Protein conformation in biomembranes: optical rotation and absorption of membrane suspensions. Biochim Biophys Acta. 1972 Feb 14;265(1):115–168. doi: 10.1016/0304-4157(72)90021-4. [DOI] [PubMed] [Google Scholar]
- Urry D. W., Trapane T. L., Walker J. T., Prasad K. U. On the relative lipid membrane permeability of Na+ and Ca2+. A physical basis for the messenger role of Ca2+. J Biol Chem. 1982 Jun 25;257(12):6659–6661. [PubMed] [Google Scholar]
- Urry D. W., Venkatachalam C. M., Spisni A., Bradley R. J., Trapane T. L., Prasad K. U. The malonyl gramicidin channel: NMR-derived rate constants and comparison of calculated and experimental single-channel currents. J Membr Biol. 1980 Jun 30;55(1):29–51. doi: 10.1007/BF01926368. [DOI] [PubMed] [Google Scholar]
- Veatch W. R., Durkin J. T. Binding of thallium and other cations to the gramicidin A channel. Equilibrium dialysis study of gramicidin in phosphatidylcholine vesicles. J Mol Biol. 1980 Nov 15;143(4):411–417. doi: 10.1016/0022-2836(80)90220-x. [DOI] [PubMed] [Google Scholar]


