Abstract
Interleukin-18 (IL-18) and IL-12 play a critical role in the expression of cell-mediated immunity involved in host defense against intracellular pathogens. Both cytokines are produced by macrophages and act in synergy to induce gamma interferon (IFN-γ) production by T, B, and natural killer cells. In the present study, we analyzed both cellular and humoral responses upon infection with IL-18-secreting BCG of BALB/c and C3H/HeJ mice, two strains known to differ in their ability to support the growth of BCG. The cDNA encoding mature IL-18 was fused in frame with the alpha-antigen signal peptide-coding sequence, cloned downstream of the mycobacterial hsp60 promoter and expressed in BCG. IL-18 produced by the recombinant BCG strain was functional, as judged by NF-κB-mediated luciferase induction in a tissue culture assay. When susceptible mice were infected with IL-18-producing BCG, their splenocytes were found to produce higher amounts of Th1 cytokines after stimulation with mycobacterial antigens than the splenocytes of mice infected with the nonrecombinant BCG. This was most prominent for IFN-γ, although the mycobacterial antigen-specific secretion of granulocyte-macrophage colony-stimulating factor and IL-10 was also augmented after infection with the recombinant BCG compared to infection with nonrecombinant BCG. In contrast, the immunoglobulin G levels in serum against mycobacterial antigens were lower when the mice were infected with IL-18-producing BCG compared to infection with nonrecombinant BCG. The IL-18 effect was delayed in BALB/c compared to C3H/HeJ mice. These results indicate that the production of IL-18 by recombinant BCG may enhance the immunomodulatory properties of BCG further toward a Th1 profile. This may be particularly useful for immunotherapeutic or prophylactic interventions in which a Th1 response is most desirable.
Mycobacterium bovis BCG induces a characteristic Th1-type immune response upon infection of the host macrophages (15, 30). Among the Th1 cytokines that are induced by BCG infection, gamma interferon (IFN-γ) plays a major role in the activation of cell-mediated immunity (7, 9, 16, 20, 39).
Because of its strong immunostimulatory properties, BCG treatment may have profound effects on the outcome of a variety of diseases in which the Th1/Th2 balance plays an important role. In addition to its use as a vaccine against tuberculosis, it has been shown to be the most effective intravesical agent for the treatment of superficial bladder cancer and prophylaxis against recurrent disease (18, 27, 31, 36). Bladder cancer patients that respond to BCG treatment produce massive amounts of urinary IFN-γ, together with other Th1 cytokines, whereas high levels of Th2 cytokines are produced by patients that fail to respond to BCG (26), suggesting a favorable effect of Th1 type cytokines on the efficiency of the cancer treatment.
In atopic diseases, such as asthma, the Th1/Th2 balance also appears to play a critical role. The processes leading to allergic inflammation are induced by Th2 lymphocytes (6, 14). Children vaccinated with BCG and who develop a positive tuberculin skin test, associated with a higher Th1/Th2 ratio, have been found to less likely develop atopic disease compared to tuberculin skin test-negative children (45). Since IFN-γ is a powerful suppressive mediator of Th2 activity, BCG injections, leading to an enhancement of IFN-γ production (39), appear to help in resolving atopic symptoms. Similarly, BCG infection has been demonstrated to suppress the development of lung inflammatory Th2 responses in a murine model of allergen-induced airway eosinophilia (13).
Although the immunomodulatory properties of BCG and their effects on the modulation of the pathogenesis of a variety of diseases have been well established both in humans and in animal models, the magnitude of the response to BCG may vary substantially between individuals. The reasons for these variations are not known but may be related to differences between individuals in the genetic background, in the general physiological status, or in the immunological history of encounter with infectious agents. In certain cases, immunomodulation offered by BCG may simply not be strong enough. Increasing the immunomodulatory potential of BCG may therefore have important implications in the treatment of a number of different diseases.
IFN-γ production is induced by interleukin-18 (IL-18) in synergy with IL-12 (10, 38). Since mycobacterial infections result in the secretion of IL-12 (8), which subsequently may lead to the production of IFN-γ, we reasoned that infection with recombinant mycobacteria producing IL-18 may perhaps further increase the IFN-γ production. In the present study we therefore constructed a recombinant (rBCG) strain producing murine IL-18 (mIL-18). We show here that the long-term production of IFN-γ is strongly augmented, whereas the production of antibodies is decreased in two different inbred mouse strains, indicating that the production of mIL-18 by BCG may strongly enhance its ability to polarize the immune response into the Th1 direction.
MATERIALS AND METHODS
Plasmids and DNA manipulation.
Plasmids pUC::hsp60 (21) and pEN103 (4) have been described previously, and pENH-TK (1) was kindly provided by C. Grangette (Institut Pasteur, Lille, France) and J.-L. Virelizier (Institut Pasteur, Paris, France). Restriction enzymes and T4 DNA ligase were purchased from Boehringer Mannheim GmbH (Mannheim, Germany). All DNA manipulations were performed by using standard protocols as described previously (41).
Bacterial strains and growth conditions.
All cloning steps were performed in Escherichia coli XL1-Blue (Stratagene, La Jolla, Calif.). For the production of recombinant mIL-18 we used the M. bovis BCG vaccine strain 1173P2 (World Health Organization, Stockholm, Sweden). BCG was transformed as previously described (21), and rBCG clones were selected on Middlebrook 7H10 agar supplemented with oleic acid-albumin-dextrose-catalase enrichment (Difco, Detroit, Mich.) and 10 μg of HgCl2/ml. Liquid cultures of rBCG were grown at 37°C in Sauton medium (43) containing 8 μg of HgCl2/ml by using stationary tissue culture flasks.
Viable BCG counts.
To count viable BCG in mouse organ homogenates, groups of four BALB/c and C3H/HeJ mice were killed at different time points after a single intraperitoneal (i.p.) administration of ca. 5 × 106 BCG or rBCG. The spleens were homogenized separately in 5 ml of RPMI 1640 medium, and serial dilutions were plated onto Middlebrook 7H10 medium. Viable CFU were counted after a 3-week incubation at 37°C.
Construction of the mIL-18 expression vector.
The 491-bp cDNA fragment encoding the mature portion of mIL-18 was amplified by PCR as described previously (22) and then restricted by BglII and Asp718. The 126-bp DNA fragment encoding the alpha-antigen signal sequence was amplified by PCR with the primers 5′-GGCACAGGTCATGACAGACGTGAGCCGAAAGATTCGA-3′ and 5′-GCCGGGATCCCGCGCCCGCGGTTGCCGCTCCGCC-3′ (Eurogentec, Liège, Belgium) and was restricted by using BspHI and BamHI corresponding to the sequences underlined. The two PCR fragments were then cloned into pUC::hsp60 restricted by NcoI and Asp718, thereby generating pUC::IL-18. The 1-kb KpnI/HindIII fragment from pUC::IL-18 spanning the BCG hsp60 promoter, the ribosomal binding site, the alpha-antigen signal peptide coding sequence, and the mature IL-18 coding sequence was cloned into pEN103 previously digested with KpnI and HindIII. The resulting shuttle vector, pENIL-18, was used to transform BCG, and the transformants were selected by their resistance to HgCl2 as described previously (2). HgCl2-resistant BCG colonies were analyzed for their plasmid content by using electroduction (3).
Detection of mIL-18 in recombinant mycobacteria.
Mycobacterial cell extracts were prepared as described previously (24) from 10-ml cultures harvested at mid-log phase. The proteins in the lysates, corresponding to ca. 5 × 106 bacteria, were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 15% gel as described previously (25). Total proteins were then transferred onto a Hybond-C extra membrane (Amersham France). The membrane was saturated with 1% bovine serum albumin in phosphate-buffered saline (PBS)-0.1% Tween 20 (PBST) and then incubated with rabbit anti-IL-18 antiserum diluted 1/2,000 (22). Goat anti-rabbit alkaline phosphatase-conjugated antibodies (Promega, Madison, Wis.) diluted 1/7,000 in PBST were then used to develop the immunoblots.
Luciferase cell assay.
The activity of the recombinant IL-18 was assessed by using a U937 human monocyte cell line transfected with a luciferase-encoding reporter cassette that contains three copies of the synthetic human immunodeficiency virus enhancer oligonucleotide with the NF-κB consensus motif cloned upstream of the thymidine kinase promoter that controls the luciferase gene transcription (1). Activation of NF-κB in this cell line results in luciferase production (1, 44). The transfected cells were stimulated by the presence of either 2 μg of phytohemagglutinin (PHA) or 1 μg of lipopolysaccharide (LPS)/ml or by infection with nonrecombinant BCG or mIL-18-producing BCG in the presence or absence of rabbit anti-IL-18 antiserum diluted 1/50. After 16 h of stimulation, the cells were centrifuged for 5 min at 1,600 rpm. The supernatants were removed, and the cells were lysed with 100 μl of Luciferin buffer (LB) containing 25 mM Tris phosphate (pH 7.8), 8 mM MgCl2, 1 mM dithiothreitol, 1% Triton X-100, 1% bovine serum albumin, and 15% glycerol. The lysates were clarified by centrifugation for 5 min at 2,000 rpm and diluted in 150 μl of LB in a scintillation vial. Then, 4 μl of 25 mM ATP (Sigma) and 20 μl of 1 mM Luciferin (Sigma) were added, followed by thorough mixing. The luciferase activity was assayed by using a standard scintillation counter (Nucleotimetre 107; Interbio, Berthold, Germany). The background activity, determined with 150 μl of LB, ATP, and Luciferin in the absence of cell extracts, was subtracted from all sample values.
Immunization protocol.
Groups of 18 6-week-old female BALB/c (H-2d) and C3H/HeJ (H-2k) mice (Iffa Credo, l'Arbresle, France) were immunized i.p. with 5 × 106 nonrecombinant BCG or BCG producing mIL-18 in a final volume of 100 μl. A control group received 100 μl of PBS.
RNA extraction.
Spleen tissue samples were taken from infected mice, mixed in RNAzol (Appligene Oncor), and stored at −80°C until further use. Total RNA was extracted from 106 spleen cells. Reverse transcription-PCR (RT-PCR) was carried out as described previously (22) with gene-specific primer sets for IFN-γ (sense, 5′-GTCTGAAGAACTATTTTAACTCAAG, antisense, 5′-GTGGGTTGTTCACCTCGAACTTGG; size of product, 279 bp), IL-12p40 (sense, 5′-GACCCTGCCCATTGAACTGGC; antisense, 5′-CAACGTTGCATCCTAGGATCG; size of product, 415 bp), IL-13 (sense, CTCCCTCTGACCCTTAAGGAG; 5′ antisense, 5′-GAAGGGGCCGTGGCGAAACAG; size of product, 309 bp), IL-4 (sense, 5′-ACGAGGTCACAGGAGAAGGGACGCCATGCA; antisense, 5′-TCATTCATGGAGCAGCTTATCGATGAATCC; size of product, 188 bp), and IL-5 (sense, 5′-AGGATGCTTCTGCACTTGAGTGTTC; antisense, 5′-GCCTTCCATTGCCCACTCTGTACTC; size of product, 393). The β-actin cDNA with a size of 540 bp used as an internal control was amplified by RT-PCR from the spleen tissues with the primers sense (5′-GTGGGGCGCCCCAGGCACCA) and antisense (5′-CTCCTTAATGTCACGCACGATTTC). The PCR products were analyzed by agarose gel electrophoresis and ethidium bromide staining.
Lymphocyte proliferation and cytokine and antibody assays.
Mice were sacrificed 21, 50, and 120 days after infection. For each time point, four to six mice were used. Spleens were removed aseptically and placed in RPMI 1640 medium (Gibco, Courbevoie, France) containing 10% fetal calf serum (Boehringer, Meylan, France), 50 μM 2-mercaptoethanol (Merck, Darmstadt, Germany), 30 μg of glutamine/ml, 50 μg of penicillin (Gibco)/ml, and 50 μg of streptomycin (Gibco)/ml. Single-cell suspensions were prepared and incubated at a concentration of 5 × 105 cells/well in 96-well plates (Falcon; Becton Dickinson, Mountain View, Calif.) in the presence of various concentrations of purified protein derivative (PPD; Denmark Serum Institute, Copenhagen, Denmark) or 5 μg of concanavalin A (ConA; Sigma)/ml. To measure lymphoproliferation, the cells were pulsed for 18 h with 1 μCi of [3H]thymidine as described previously (23). The cells were then harvested, and thymidine incorporation was measured by using a beta counter (LKB Wallac, St. Quentin, France). The cell culture supernatants were collected after 72 h of PPD or ConA stimulation and assessed for the presence of cytokines by sandwich enzyme-linked immunosorbent assay (ELISA). The antibodies used for the detection were purchased from PharMingen (San Diego, Calif.) and used as recommended by the supplier. These antibodies were the anti-IFN-γ antibody (clone R4-6A2 for capture and SMG1-2 for detection; sensitivity, 20 pg/ml), the anti-granulocyte-macrophage colony-stimulating factor antibody (GM-CSF; clone MP1-22E9 for capture and MP1-31G6 for detection; sensitivity, 60 pg/ml), the anti-IL-4 antibody (clone 11B11 for capture and MP5-32C11 for detection; sensitivity, 10 pg/ml), the anti-IL-6 antibody (clone MP5-20F3 for capture and SMG1-2 for detection; sensitivity, 50 pg/ml), the anti-IL-10 antibody (clone JES5-2A5 for capture and SXC-1 for detection; sensitivity, 150 pg/ml), and the anti-IL-12 antibody (clone C8.3 for capture and C17.8 for detection; sensitivity, 150 pg/ml) antibodies. For the detection of anti-IL-13 antibodies, clone 38313.11 for capture and biotinylated polyclonal antibodies for detection (sensitivity, 50 pg/ml) were used (R&D Systems, Minneapolis, Minn.). Tumor necrosis factor alpha (TNF-α) was measured by using the mouse TNF-α Quantikine immunoassay kit (R&D Systems). Optical densities were measured at 492 nm by using a multichannel spectrophotometer (Titertek Multiskan MCC 1340).
The ELISAs used for the detection of anti-BCG antibodies, as well as the preparation of soluble BCG antigens, were described elsewhere (24).
Statistical significance was estimated by using the Mann-Whitney test (P < 0.05).
RESULTS
Production and secretion of mIL-18 by rBCG.
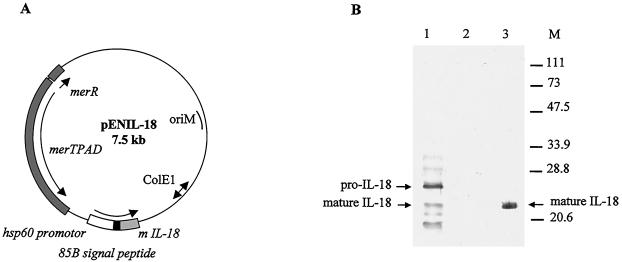
To construct a BCG strain able to produce and secrete mIL-18, the mIL-18-encoding cDNA was prepared as described previously (22) and was modified by substituting the original IL-18 signal peptide coding sequence with the mycobacterial secretion signal sequence from the BCG alpha-antigen (34). The modified mIL-18 cDNA was inserted into the expression vector pEN103 (4) (Fig. 1A) and then introduced into BCG. The rBCG were analyzed by immunoblotting with an anti-IL-18 rabbit antiserum (22). As shown in Fig. 1B, several immunoreactive proteins were present in the lysate of rBCG producing mIL-18, but not in the lysate of the nonrecombinant control strain. The presence of an immunoreactive protein with a size similar to that of purified IL-18 suggests that at least for some recombinant mIL-18 molecules the signal peptide had been cleaved off. The immunoreactive protein of a higher molecular weight has a size corresponding to pro-mIL-18 that still contains the alpha-antigen signal peptide. Several smaller peptides detected in the rBCG strain most likely correspond to breakdown products. These results show that the hsp60 promoter is able to drive expression of the pro-mIL-18 in BCG and that the recombinant IL-18 is at least partially converted into the secreted mature form.
FIG. 1.
Production of mIL-18 by rBCG. (A) Construction of pENIL-18 used to produce and secrete mIL-18 by BCG. The plasmid contains a mercury resistance operon (merTPAD) used as a selective marker. ColE1 represents the origin of replication from pUC18, and oriM represents the mycobacterial origin of replication. The expression cassette contains the BCG hsp60 promoter, the ribosome-binding site and the hsp60 initiating codon represented by the white box. The M. tuberculosis alpha-antigen signal peptide coding sequence is represented by the black box, whereas the IL-18 coding sequence is shown in gray. (B) Immunoblot analysis of rBCG producing mIL-18. Whole-cell extracts of recombinant (lane 1) or untransformed (lane 2) BCG, each corresponding to an optical density at 600 nm of 0.2 were analyzed by immunoblotting with rabbit anti-IL-18 antibodies. Lane 3 contains 100 ng of purified mature IL-18 produced by E. coli. The sizes of the molecular weight markers are indicated in the right margin.
Functional analysis of the recombinant mIL-18 produced by BCG.
To investigate the functionality of the mIL-18 produced by BCG, we used an assay based on the induction of a NF-κB-depending luciferase reporter gene. Since IL-18 is known to activate NF-κB (48), the induction of NF-κB-dependent genes in a monocyte cell line may reflect the functionality of IL-18. As shown in Table 1, U937 monocytes transfected with the NF-κB-dependent luciferase expression vector pENH-TK (1) and activated with LPS or PHA produced increased luciferase activity, whereas infection with the nonrecombinant BCG strain did not induce significant activity. In contrast, when the cells were infected with the recombinant strain producing mIL-18, high levels of luciferase activity were detected. This activity was at least twofold higher than that of cells activated with PHA and was abolished in the presence of anti-IL-18 antibodies. These results indicate that the mIL-18 produced by the rBCG is able to activate NF-κB, implying thereby that it is efficiently processed into a functionally active form.
TABLE 1.
Luciferase activity in U937 cells transfected with pENH-TK
| Stimulusa | Mean luciferase activity (LIb ± SD) |
|---|---|
| PHA | 3.25 ± 0.55 |
| LPS | 1.75 ± 0.28 |
| BCG NR | 0.9 ± 0.52 |
| BCG IL-18 | 7.25 ± 4.8 |
| BCG IL-18 + IL-18-antiserum | 1.31 ± 0.77 |
U937 transfected with pENH-TK were plated in 96-well plates and stimulated for 20 h with PHA, LPS, nonrecombinant BCG, or IL-18-secreting BCG as described in Materials and Methods. Triplicates of each sample were used to determine luciferase activity as expressed by the luciferase index (LI).
Results are expressed as the mean LI ± SD obtained from three independent assays. Activities were calculated as follows: LI = [Luc activity in sample (cpm) − background Luc activity (cpm)]/[Luc activity in nonstimulated sample (cpm) − background Luc activity (cpm)], where Luc is luciferase and cpm is the counts per minute.
Kinetics of bacterial growth.
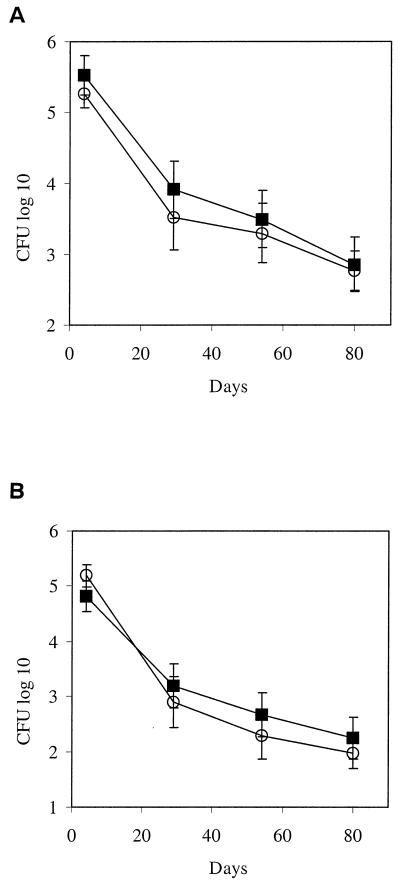
Growth of rBCG was compared to that of nonrecombinant BCG in the spleens of BALB/c and C3H/HeJ mice after a single i.p. administration. The results shown in Fig. 2 indicate that the persistence of rBCG is very similar to that observed with the control BCG in BALB/c mice (Fig. 2A), as well as in C3H/HeJ mice (Fig. 2B). The number of CFU in the spleens of the mice decreased regularly over an 80-day observation period. The clearance of either BCG strain was faster in C3H/HeJ mice than in BALB/c mice.
FIG. 2.
Colonization of mouse spleens by IL-18-producing BCG versus nonrecombinant BCG. After a single i.p. administration of 5 × 106 rBCG (○) or nonrecombinant BCG (▪), BALB/c (A) and C3H/HeJ (B) mice were sacrificed at the indicated time points. The spleens were removed and homogenized, and the individual organ extracts were plated onto Middlebrook 7H10 medium supplemented with OADC (oleic acid-albumin-dextrose-catalase). BCG colonies were counted after 3 weeks of incubation at 37°C. The SD values were calculated from groups of four animals for each time point.
Cell-mediated immune responses in BALB/c mice immunized with mIL-18-secreting BCG.
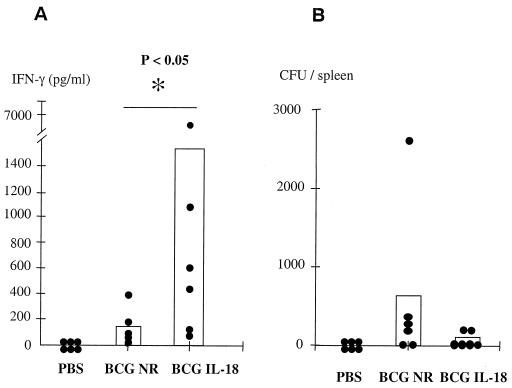
IL-18 has been reported to promote a strong Th1 response characterized by the induction of IFN-γ (38). To investigate whether the rBCG producing mIL-18 was able to generate an antigen-specific Th1-type response, mice were primed i.p. with either 5 × 106 CFU of nonrecombinant BCG or rBCG. Previous studies conducted by Murray et al. (37) have shown that a strong cell-mediated immune response in mice infected with BCG producing murine cytokines appeared several months after immunization. We therefore analyzed the cellular response 120 days after immunization. Splenocytes were isolated from BALB/c mice, cultured individually, restimulated in vitro with PPD, and assayed for the secretion of IFN-γ. No IFN-γ production (<30 pg/ml) could be detected in the supernatants of the cells isolated from mice treated with PBS and stimulated in vitro with PPD (Fig. 3A). In contrast, the splenocytes from mice immunized with nonrecombinant BCG produced significant amounts of IFN-γ (123 pg/ml; standard deviation [SD], ±59) upon stimulation with PPD. When the mice had been immunized with the mIL-18-producing strain, the IFN-γ production by their splenocytes, restimulated in vitro with PPD, was substantially enhanced (1,522 pg/ml; SD, ±1,068). This marked increase in IFN-γ secretion in response to PPD indicates that the rBCG strain secretes functionally active mIL-18 that is able to modulate the immune response toward the Th1 type. During the time course of this experiment, the persistence of the two BCG strains was evaluated by counting the CFU in the spleen of individual immunized mice. Very similar bacterial loads were present 120 days after immunization, regardless of the BCG strain administered (Fig. 3B). Similar results were obtained when lymph nodes were analyzed. These observations indicate that the increase in IFN-γ production observed in the mice immunized with the mIL-18-producing strain is mediated by the immunostimulatory properties of the active mIL-18 rather than by a difference in bacterial load or in persistence or clearance of the two strains.
FIG. 3.
In vitro IFN-γ production by splenocytes in response to PPD. BALB/c mice were immunized with 5 × 106 BCG, and 120 days later the spleens from individual mice (six mice per group) were harvested to measure PPD-specific IFN-γ secretion (A) and bacterial counts (B). To measure the IFN-γ secretion, the splenocytes were restimulated in vitro with 25 μg of PPD/ml, the supernatants were then recovered after 72 h of PPD stimulation and assayed by ELISA for IFN-γ production. IFN-γ secretion by unstimulated cells were below detectable levels. Each point corresponds to the mean of triplicate measurements.
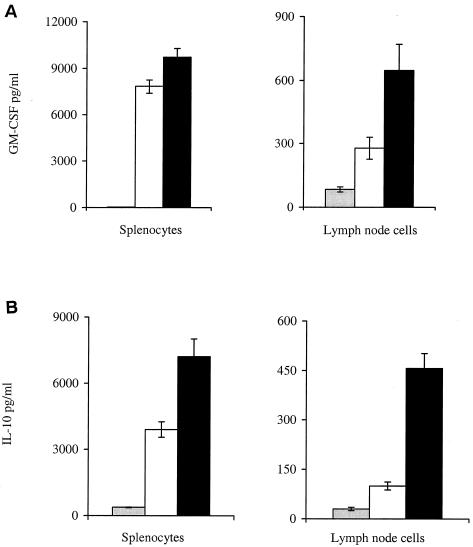
In addition to IFN-γ, PPD-stimulated splenocytes and PPD-stimulated lymph node cells isolated from mice infected with mIL-18-secreting BCG produced also increased the levels of GM-CSF and IL-10, compared to cells isolated from mice infected with nonrecombinant BCG (Fig. 4). The amounts of IL-4, IL-5, IL-6, IL-12, IL-13 and TNF-α were below detectable levels in both groups of mice (data not shown).
FIG. 4.
GM-CSF and IL-10 production by PPD-stimulated splenocytes and lymph node cells. BALB/c mice were infected i.p. with 5 × 106 BCG producing IL-18 (▪) or 5 × 106 nonrecombinant BCG (□) or were treated with PBS ( ). The splenocytes and lymph node cells were isolated 120 days later and restimulated in vitro with 25 μg of PPD/ml, pooled (six mice per group), and assayed by ELISA for the production of GM-CSF (A) and IL-10 (B). Each culture condition was done in triplicate.
Cytokine induction in vivo in BALB/c mice.
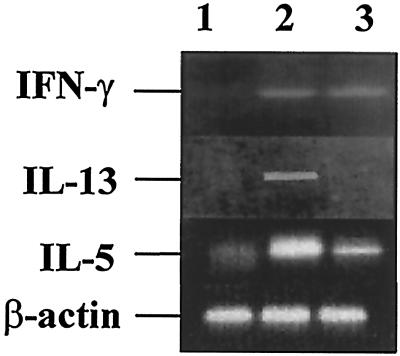
As a more sensitive assay for the detection of Th2-type cytokines, we performed RT-PCR on RNA isolated from spleen cells of mice 120 days after one injection of 5 × 106 BCG. As shown in Fig. 5, the levels of mRNA encoding IL-13 and IL-5 were significantly reduced in mice primed with rBCG compared to mice infected with nonrecombinant BCG. In contrast, the levels of mRNA specific for IFN-γ were found to be substantially higher in mice infected with BCG compared to mice treated with PBS. However, there was no significant difference between the mice that had received mIL-18-producing BCG and nonrecombinant BCG. These measurements have been done twice with the same results.
FIG. 5.
Modulation of IL-13 and IL-5 mRNA production by BCG producing mIL-18. The production of mRNA for IFN-γ, IL-13, and IL-5 was analyzed by RT-PCR from pooled unstimulated splenocytes prepared from BALB/c mice 120 days after treatment with PBS (lanes 1) or with either nonrecombinant BCG (lanes 2) or IL-18-secreting BCG (lanes 3). The bottom lanes correspond to the amplification with β-actin mRNA-specific primers as control.
IFN-γ could not be detected in serum in any experimental group. Together, these results indicate that in the absence of stimulation by mycobacterial antigens, mIL-18-producing BCG does not induce non-antigen-specific Th1-type cytokines, such as IFN-γ, whereas it downregulates the production of Th2-type cytokines, such as IL-13 and IL-5.
IFN-γ response in C3H/HeJ mice immunized with mIL-18-producing-BCG.
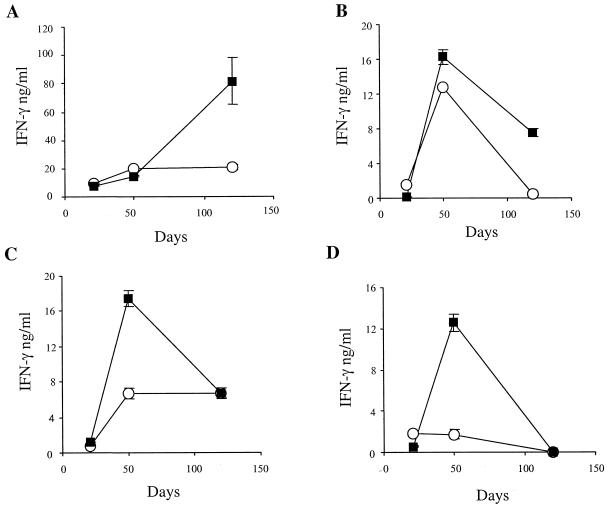
To investigate whether the BCG-IL-18 effect was specific for a single inbred mouse strain, we evaluated the effect of the mIL-18-producing BCG strain on the immune response of a different mouse strain, C3H/HeJ. As shown in Fig. 2 the clearance of the BCG strains was more rapid in C3H/HeJ than in BALB/c mice, which could potentially affect the immune responses. Antigen-specific responses were thus compared in both BALB/c and C3H/HeJ mice primed with BCG producing mIL-18. The kinetics of IFN-γ production were assessed in supernatants from splenocytes (Fig. 6A and C) and lymph node cells (Fig. 6B and D) restimulated in vitro with PPD. In the supernatants of cells isolated from C3H/HeJ mice immunized with the recombinant strain, IFN-γ production increased, peaked 50 days after immunization and then started to decline to reach the same level as for C3H/HeJ mice immunized with the nonrecombinant strain (Fig. 6C and D). In contrast, although a peak of IFN-γ production was observed at day 50 for the lymph node cells of BALB/c mice (Fig. 6B), different kinetics of IFN-γ secretion were observed in the spleen, with the highest response at day 120 (Fig. 6A). In addition, the intensity of the response in the spleen of BALB/c mice was much stronger than that seen in the spleen of C3H/HeJ mice.
FIG. 6.
In vitro IFN-γ secretion in response to PPD. Splenocytes (A and C) or lymph node cells (B and D) from five BALB/c (A and B) or C3H/HeJ (C and D) mice were harvested 21, 50, or 120 days after injection of nonrecombinant BCG (○) or IL-18-producing BCG (▪) and stimulated with PPD before the IFN-γ secretion was measured. The results are expressed as the means of triplicates with SDs.
The enhanced IFN-γ production by splenocytes or by lymph node cells induced by the rBCG strain might result from proliferation of antigen-specific IFN-γ-secreting cells or from an increase in the amounts of IFN-γ produced per cell. To discriminate between these two possibilities, we compared the proliferative response to PPD stimulation of splenocytes and lymph node cells from mice immunized with BCG producing IL-18 versus nonrecombinant BCG 120 days after immunization. No difference in thymidine uptake by the splenocytes or lymph node cells in response to PPD stimulation was observed between the two groups. These observations indicate that the effect of BCG producing IL-18 on IFN-γ production is not the result of an increase in the numbers of antigen-specific IFN-γ-secreting cells but is due to an increase in IFN-γ-secreting activity of a subset of specific T lymphocytes.
Humoral immune responses induced by BCG producing IL-18.
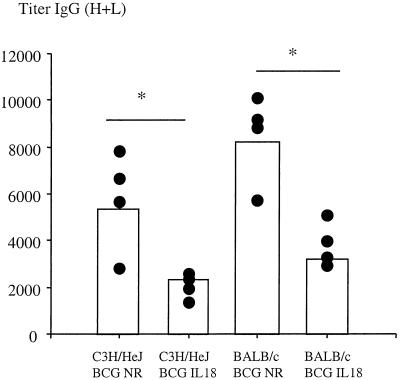
Concomitant with the cell-mediated immune responses, the kinetics of the humoral responses directed against total soluble BCG antigens were determined in both BALB/c and C3H/HeJ mice. The results shown in Fig. 7 indicate that the total immunoglobulin G response was approximately threefold lower in mice immunized with mIL-18-secreting BCG compared to mice immunized with nonrecombinant BCG, regardless of the mouse strain. Since similar bacterial loads were found in mice immunized with either BCG strain, it follows that mIL-18 produced by rBCG negatively affects antigen-specific antibody production in both mouse strains.
FIG. 7.
Anti-BCG antibody responses. BALB/c mice and C3H/HeJ mice were infected i.p. with nonrecombinant BCG (BCG NR) or IL-18-producing BCG (BCG IL-18) and bled 120 days after infection. Anti-BCG IgG titers of individual mice were determined by ELISA. The titers are defined as the maximal serum dilutions that gave absorbancies that were threefold higher than the background level. Values represent antibody titers in serum 120 days after infection from four individual mice. ✽, P < 0.05.
DISCUSSION
IL-18, originally designated IFN-γ-inducing factor, is an important immune regulator implicated in both innate and acquired immune responses (11, 38). IL-18 has been shown to play a central role in the development of immunity against pathogens, by acting on Th1 cell differentiation, cell-mediated cytotoxicity, and inflammation via IFN-γ production. Its role in protective immunity to intracellular parasites, including Salmonella enterica serovar Typhimurium, Mycobacterium tuberculosis, Shigella flexneri, and Chlamydia trachomatis has been well documented (5, 12, 29, 32, 42, 46, 47). We have previously shown that mice infected with BCG after intradermal administration of DNA encoding IL-18 led to a reduction in the humoral immune responses against BCG and a marked increase of antigen-specific IFN-γ secretion (22). Because of its involvement in the very early steps of the cytokine cascade leading to a Th1 type immune response, IL-18 may constitute an ideal cytokine candidate to be used for directing the immune response toward the Th1 type. IL-18 acts in synergy with IL-12 to induce the production of IFN-γ. Since infection with mycobacteria usually results in the secretion of large amounts of IL-12 (8), the coadministration of IL-18 and mycobacteria, for example, in the form of rBCG producing IL-18, may result in a synergistic effect leading to high levels of IFN-γ production.
In this report we show that active mIL-18 was produced and secreted by rBCG when the mIL-18 cDNA was placed under the control of the hsp60 promoter, and secretion was driven by the signal peptide derived from the M. tuberculosis alpha-antigen. In its natural environment, IL-18 is produced as a precursor containing a leader peptide that requires processing by the IL-1β converting enzyme (17). Only the matured form is biologically active. Since BCG does not produce IL-1β-converting enzyme, we chose to fuse the mycobacterial alpha-antigen signal peptide to the mIL-18 portion that corresponds to the mature protein. As evidenced by immunoblot analysis, the chimeric pro-mIL-18 was converted at least partly into the mature cytokine form, although a substantial amount of the protein remained in the form of pro-mIL-18. More efficient processing may require some additional engineering at the signal peptide cleavage site. Nevertheless, the BCG-produced mIL-18 was biologically active, as shown in an in vitro assay using a luciferase reporter gene, the expression of which is NF-κB dependent. Binding of IL-18 to its receptor induces the recruitment of serine-threonine IL-1 receptor-associated kinases to the receptor complex, which requires the MyD88 (28). This then triggers a phosphorylation cascade that ultimately leads to the release of IκB from the NF-κB-IκB complex, allowing the translocation of NF-κB into nucleus to occur (33, 40). Finally, the translocation of NF-κB into the nucleus induces the expression of the luciferase gene in the reporter system used here. We found that high levels of luciferase activity could be measured when the cells were incubated with the mIL-18-producing BCG, whereas the nonrecombinant BCG control strain did not induce the expression of the luciferase gene. Although this in vitro assay is not specific for IL-18 per se, since other cytokines may activate NF-κB, the inhibition of the rBCG-mediated luciferase induction by anti-IL-18 antibodies indicates that in this particular assay, we detect IL-18-mediated activation of NF-κB. Since the target cell was of human origin, these results also indicate that the mIL-18 is recognized by the human IL-18 receptor.
Mice immunized with BCG strains secreting mIL-18 exhibited enhanced antigen-specific IFN-γ secretion after in vitro restimulation of both splenocytes and lymph node cells with PPD. Examination of the bacterial loads showed that most of bacilli were cleared 120 days after administration, regardless of the BCG strain used. Therefore, the increased antigen-specific IFN-γ response observed is likely due to the enhanced immunostimulatory properties of the mIL-18-producing BCG compared to the nonrecombinant strain rather than to differences in the persistence of the two strains in mice. Histological analyses of the lungs, spleens, and livers did not reveal any alteration of the tissue morphology of these organs (data not shown), and mice treated with the BCG strain producing IL-18 did not present any sign of sickness.
By comparing BALB/c and C3H/HeJ mice, we found that the enhanced cellular response is not restricted to the BALB/c mice. C3H/HeJ mice, described as being relatively resistant to BCG infection, clear BCG strains more rapidly than do BALB/c mice (50). Despite these differences, both mouse strains produced an enhanced antigen-specific T-cell response. However, in C3H/HeJ mice, the enhanced IFN-γ production in the spleen was detected earlier and found to be weaker than in BALB/c mice.
In addition to IFN-γ, splenocytes and lymph node cells from mice injected with mIL-18-secreting BCG also produced higher levels of IL-10 and GM-CSF compared to the BCG controls. IL-10 is an anti-inflammatory cytokine induced as a consequence of elevated levels of IFN-γ and is believed to control the IFN-γ production, thereby avoiding excessive inflammation. Consistent with the documented ability to induce the production of GM-CSF by stimulated peripheral blood monocytes in humans (49), splenocytes isolated from mice infected with the rBCG and stimulated with PPD also showed increased GM-CSF production over cells isolated from mice infected with the nonrecombinant control strain. Several rBCG strains secreting Th1 cytokines have been shown to induce strong GM-CSF production in immunized mice (35, 37).
The induction of Th1-type cytokines mediated by mIL-18-producing BCG was detectable after in vitro stimulation with mycobacterial antigens. Compared to nonrecombinant BCG, mIL-18-producing BCG did not increase IFN-γ mRNA levels in spleen cells without in vitro stimulation by mycobacterial antigens as indicated by RT-PCR. However, even in the absence of in vitro antigen stimulation the production of mIL-18 by BCG resulted in reduced expression of Th2-type cytokine mRNAs, such as those coding for IL-5 and IL-13, indicating that, in addition to the stimulation of antigen-specific IFN-γ production, mIL-18-producing BCG downregulates the general Th2 response. Although IL-18 has been shown to upregulate Th2 cytokines such as IL-13, in the context of certain infections, these cytokines can also be downregulated by IL-18 (19). The IL-18 effect on Th2 cytokines appears thus to depend strongly on the context of infection. The downregulation of Th2 cytokines seen in the present study is consistent with the observation that the total antibody response to mycobacterial antigens was decreased in mice infected with mIL-18-secreting BCG compared to those infected with the nonrecombinant strain. A similar effect has also been observed when the IL-18 was given in the form of a recombinant IL-18-encoding plasmid, separately, of the BCG (22).
The ability of IL-18 produced by rBCG to strengthen the natural capacity of BCG to induce Th1-type immune responses may have interesting therapeutic or immunoprophylactic potential. It may have a positive impact on vaccination strategies, especially against intracellular pathogens, including M. tuberculosis, which is still the world's leading cause of death due to a single infectious agent. In addition, IL-18 may increase the therapeutic effect of BCG in the treatment of bladder cancer due to its ability to help BCG in inducing local IFN-γ production. Importantly, the shuttle vector pENIL-18 contains mercury resistance determinants as the only selectable marker (3, 24). The absence of antibiotic resistance markers in this vector avoids the possible dissemination of antibiotic resistance if the strain were to be used in human or animal therapy. Finally, because its ability to downregulate Th2 type immune responses, BCG producing IL-18 may also find useful applications in pathologies caused by the exacerbation of Th2 responses, such as in allergic reactions and asthma. Independently, both BCG (13) and IL-18 (19) have already been reported to suppress atopic disorders. The combination of both in the form of an rBCG strain may perhaps enhance these therapeutic effects.
Acknowledgments
We thank J. L. Virelizier and C. Grangette for the generous gift of pENH-TK.
This work was supported by the Institut Pasteur de Lille, INSERM, Région Nord-Pas de Calais, and EC grant QLRT-PL 1999-01093.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Bachelerie, F., J. Alcami, F. Arenzana-Seisdedos, and J. L. Virelizier. 1991. HIV enhancer activity perpetuated by NF-κB induction on infection of monocytes. Nature 350:709-712. [DOI] [PubMed] [Google Scholar]
- 2.Baulard, A., V. Escuyer, N. Haddad, L. Kremer, C. Locht, and P. Berche. 1995. Mercury resistance as a selective marker for recombinant mycobacteria. Microbiology 141:1045-1050. [DOI] [PubMed] [Google Scholar]
- 3.Baulard, A., C. Jourdan, A. Mercenier, and C. Locht. 1992. Rapid mycobacterial plasmid analysis by electroduction between Mycobacterium spp. and Escherichia coli. Nucleic Acids Res. 20:4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baulard, A., L. Kremer, P. Supply, D. Vidaud, J. M. Bidart, D. Bellet, and C. Locht. 1996. A new series of mycobacterial expression vectors for the development of live recombinant vaccines. Gene 176:149-154. [DOI] [PubMed] [Google Scholar]
- 5.Biet, F., C. Locht, and L. Kremer. 2002. Immunoregulatory functions of interleukin 18 and its role in defense against bacterial pathogens. J. Mol. Med. 80:147-162. [DOI] [PubMed] [Google Scholar]
- 6.Carballido, J. M., N. Carballido-Perrig, G. Terres, C. H. Heusser, and K. Blaser. 1992. Bee venom phospholipase A2-specific T-cell clones from human allergic and non-allergic individuals: cytokine patterns change in response to the antigen concentration. Eur. J. Immunol. 22:1357-1363. [DOI] [PubMed] [Google Scholar]
- 7.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper, A. M., J. Magram, J. Ferrante, and I. M. Orme. 1997. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J. Exp. Med. 186:39-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalton, D. K., S. Pitts-Meek, S. Keshav, I. S. Figari, A. Bradley, and T. A. Stewart. 1993. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science 259:1739-1742. [DOI] [PubMed] [Google Scholar]
- 10.Dinarello, C. A. 1999. Interleukin-18. Methods 19:121-132. [DOI] [PubMed] [Google Scholar]
- 11.Dinarello, C. A., D. Novick, A. J. Puren, G. Fantuzzi, L. Shapiro, H. Muhl, D. Y. Yoon, L. L. Reznikov, S. H. Kim, and M. Rubinstein. 1998. Overview of interleukin-18: more than an interferon-gamma inducing factor. J. Leukoc. Biol. 63:658-664. [PubMed] [Google Scholar]
- 12.Dybing, J. K., N. Walters, and D. W. Pascual. 1999. Role of endogenous interleukin-18 in resolving wild-type and attenuated Salmonella typhimurium infections. Infect. Immun. 67:6242-6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erb, K. J., J. W. Holloway, A. Sobeck, H. Moll, and G. Le Gros. 1998. Infection of mice with Mycobacterium bovis Bacillus Calmette-Guerin (BCG) suppresses allergen-induced airway eosinophilia. J. Exp. Med. 187:561-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erb, K. J., and G. Le Gros. 1996. The role of Th2 type CD4+ T cells and Th2 type CD8+ T cells in asthma. Immunol. Cell. Biol. 74:206-208. [DOI] [PubMed] [Google Scholar]
- 15.Fine, P. E. 1989. The BCG story: lessons from the past and implications for the future. Rev. Infect. Dis. 11(Suppl. 2):5353-5359. [DOI] [PubMed] [Google Scholar]
- 16.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu, Y., K. Kuida, H. Tsutsui, G. Ku, K. Hsiao, M. A. Fleming, N. Hayashi, K. Higashino, H. Okamura, K. Nakanishi, M. Kurimoto, T. Tanimoto, R. A. Flavell, V. Sato, M. W. Harding, D. J. Livingston, and M. S. Su. 1997. Activation of interferon-gamma inducing factor mediated by interleukin-1β converting enzyme. Science 275:206-209. [DOI] [PubMed] [Google Scholar]
- 18.Herr, H. W., V. P. Laudone, R. A. Badalament, H. F. Oettgen, P. C. Sogani, B. D. Freedman, M. R. Melamed, and W. F. Whitmore. 1988. Bacillus Calmette-Guerin therapy alters the progression of superficial bladder cancer. J. Clin. Oncol. 6:1450-1455. [DOI] [PubMed] [Google Scholar]
- 19.Hofstra, C. L., I. Van Ark, G. Hofman, M. Kool, F. P. Nijkamp, and A. J. Van Oosterhout. 1998. Prevention of Th2-like cell responses by coadministration of IL-12 and IL-18 is associated with inhibition of antigen-induced airway hyperresponsiveness, eosinophilia, and serum IgE levels. J. Immunol. 161:5054-5060. [PubMed] [Google Scholar]
- 20.Kamijo, R., J. Le, D. Shapiro, E. A. Havell, S. Huang, M. Aguet, M. Bosland, and J. Vilcek. 1993. Mice that lack the interferon-gamma receptor have profoundly altered responses to infection with Bacillus Calmette-Guerin and subsequent challenge with lipopolysaccharide. J. Exp. Med. 178:1435-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kremer, L., A. Baulard, J. Estaquier, J. Content, A. Capron, and C. Locht. 1995. Analysis of the Mycobacterium tuberculosis 85A antigen promoter region. J. Bacteriol. 177:642-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kremer, L., L. Dupre, I. Wolowczuk, and C. Locht. 1999. In vivo immunomodulation following intradermal injection with DNA encoding IL-18. J. Immunol. 163:3226-3231. [PubMed] [Google Scholar]
- 23.Kremer, L., J. Estaquier, I. Wolowczuk, F. Biet, J. C. Ameisen, and C. Locht. 2000. Ineffective cellular immune response associated with T-cell apoptosis in susceptible Mycobacterium bovis BCG-infected mice. Infect. Immun. 68:4264-4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kremer, L., G. Riveau, A. Baulard, A. Capron, and C. Locht. 1996. Neutralizing antibody responses elicited in mice immunized with recombinant bacillus Calmette-Guerin producing the Schistosoma mansoni glutathione S-transferase. J. Immunol. 156:4309-4317. [PubMed] [Google Scholar]
- 25.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 26.Lamm, D. L. 1992. Long-term results of intravesical therapy for superficial bladder cancer. Urol. Clin. North. Am. 19:573-580. [PubMed] [Google Scholar]
- 27.Lamm, D. L. 1992. Optimal BCG treatment of superficial bladder cancer as defined by American trials. Eur. Urol. 21:12-16. [DOI] [PubMed] [Google Scholar]
- 28.Liebermann, D. A., and B. Hoffman. 1998. MyD genes in negative growth control. Oncogene 17:3319-3329. [DOI] [PubMed] [Google Scholar]
- 29.Lu, H., X. Yang, K. Takeda, D. Zhang, Y. Fan, M. Luo, C. Shen, S. Wang, S. Akira, and R. C. Brunham. 2000. Chlamydia trachomatis mouse pneumonitis lung infection in IL-18 and IL-12 knockout mice: IL-12 is dominant over IL-18 for protective immunity. Mol. Med. 6:604-612. [PMC free article] [PubMed] [Google Scholar]
- 30.Luelmo, F. 1982. BCG vaccination. Am. Rev. Respir. Dis. 125:70-72. [DOI] [PubMed] [Google Scholar]
- 31.Martinez-Pineiro, J. A., J. Jimenez Leon, L. Martinez-Pineiro, L. Fiter, J. A. Mosteiro, J. Navarro, M. J. Garcia Matres, and P. Carcamo. 1990. Bacillus Calmette-Guerin versus doxorubicin versus thiotepa: a randomized prospective study in 202 patients with superficial bladder cancer. J. Urol. 143:502-506. [DOI] [PubMed] [Google Scholar]
- 32.Mastroeni, P., S. Clare, S. Khan, J. A. Harrison, C. E. Hormaeche, H. Okamura, M. Kurimoto, and G. Dougan. 1999. Interleukin 18 contributes to host resistance and gamma interferon production in mice infected with virulent Salmonella typhimurium. Infect. Immun. 67:478-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsumoto, S., K. Tsuji-Takayama, Y. Aizawa, K. Koide, M. Takeuchi, T. Ohta, and M. Kurimoto. 1997. Interleukin-18 activates NF-κB in murine T helper type 1 cells. Biochem. Biophys. Res. Commun. 234:454-457. [DOI] [PubMed] [Google Scholar]
- 34.Matsuo, K., R. Yamaguchi, A. Yamazaki, H. Tasaka, and T. Yamada. 1988. Cloning and expression of the Mycobacterium bovis BCG gene for extracellular alpha antigen. J. Bacteriol. 170:3847-3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Micallef, M. J., T. Ohtsuki, K. Kohno, F. Tanabe, S. Ushio, M. Namba, T. Tanimoto, K. Torigoe, M. Fujii, M. Ikeda, S. Fukuda, and M. Kurimoto. 1996. Interferon-gamma-inducing factor enhances T helper 1 cytokine production by stimulated human T cells: synergism with interleukin-12 for interferon-gamma production. Eur. J. Immunol. 26:1647-1651. [DOI] [PubMed] [Google Scholar]
- 36.Morales, A., and J. C. Nickel. 1992. Immunotherapy for superficial bladder cancer: a developmental and clinical overview. Urol. Clin. North. Am. 19:549-556. [PubMed] [Google Scholar]
- 37.Murray, P. J., A. Aldovini, and R. A. Young. 1996. Manipulation and potentiation of antimycobacterial immunity using recombinant bacille Calmette-Guerin strains that secrete cytokines. Proc. Natl. Acad. Sci. USA 93:934-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okamura, H., H. Tsutsi, T. Komatsu, M. Yutsudo, A. Hakura, T. Tanimoto, K. Torigoe, T. Okura, Y. Nukada, K. Hattori, et al. 1995. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature 378:88-91. [DOI] [PubMed] [Google Scholar]
- 39.Orme, I. M., P. Andersen, and W. H. Boom. 1993. T-cell response to Mycobacterium tuberculosis. J. Infect. Dis. 167:1481-1497. [DOI] [PubMed] [Google Scholar]
- 40.Robinson, D., K. Shibuya, A. Mui, F. Zonin, E. Murphy, T. Sana, S. B. Hartley, S. Menon, R. Kastelein, F. Bazan, and A. O'Garra. 1997. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-gamma production and activates IRAK and NF-κB. Immunity 7:571-581. [DOI] [PubMed] [Google Scholar]
- 41.Sambrook, J., F. E. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 42.Sansonetti, P. J., A. Phalipon, J. Arondel, K. Thirumalai, S. Banerjee, S. Akira, K. Takeda, and A. Zychlinsky. 2000. Caspase-1 activation of IL-1β and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity 12:581-590. [DOI] [PubMed] [Google Scholar]
- 43.Sauton, B. 1912. Sur la nutrition minérale du bacille tuberculeux. C. R. Hebd. Seances Acad. Sci. 155:860-861.
- 44.Schwartz, O., J. L. Virelizier, L. Montagnier, and U. Hazan. 1990. A microtransfection method using the luciferase-encoding reporter gene for the assay of human immunodeficiency virus LTR promoter activity. Gene 88:197-205. [DOI] [PubMed] [Google Scholar]
- 45.Shirakawa, T., T. Enomoto, S. Shimazu, and J. M. Hopkin. 1997. The inverse association between tuberculin responses and atopic disorder. Science 275:77-79. [DOI] [PubMed] [Google Scholar]
- 46.Sugawara, I., H. Yamada, H. Kaneko, S. Mizuno, K. Takeda, and S. Akira. 1999. Role of interleukin-18 (IL-18) in mycobacterial infection in IL-18-gene-disrupted mice. Infect. Immun. 67:2585-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takeda, K., H. Tsutsui, T. Yoshimoto, O. Adachi, N. Yoshida, T. Kishimoto, H. Okamura, K. Nakanishi, and S. Akira. 1998. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity 8:383-390. [DOI] [PubMed] [Google Scholar]
- 48.Torigoe, K., S. Ushio, T. Okura, S. Kobayashi, M. Taniai, T. Kunikata, T. Murakami, O. Sanou, H. Kojima, M. Fujii, T. Ohta, M. Ikeda, H. Ikegami, and M. Kurimoto. 1997. Purification and characterization of the human interleukin-18 receptor. J. Biol. Chem. 272:25737-25742. [DOI] [PubMed] [Google Scholar]
- 49.Ushio, S., M. Namba, T. Okura, K. Hattori, Y. Nukada, K. Akita, F. Tanabe, K. Konishi, M. Micallef, M. Fujii, K. Torigoe, T. Tanimoto, S. Fukuda, M. Ikeda, H. Okamura, and M. Kurimoto. 1996. Cloning of the cDNA for human IFN-gamma-inducing factor, expression in Escherichia coli, and studies on the biologic activities of the protein. J. Immunol. 156:4274-4279. [PubMed] [Google Scholar]
- 50.Vidal, S. M., D. Malo, K. Vogan, E. Skamene, and P. Gros. 1993. Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell 73:469-485. [DOI] [PubMed] [Google Scholar]