Abstract
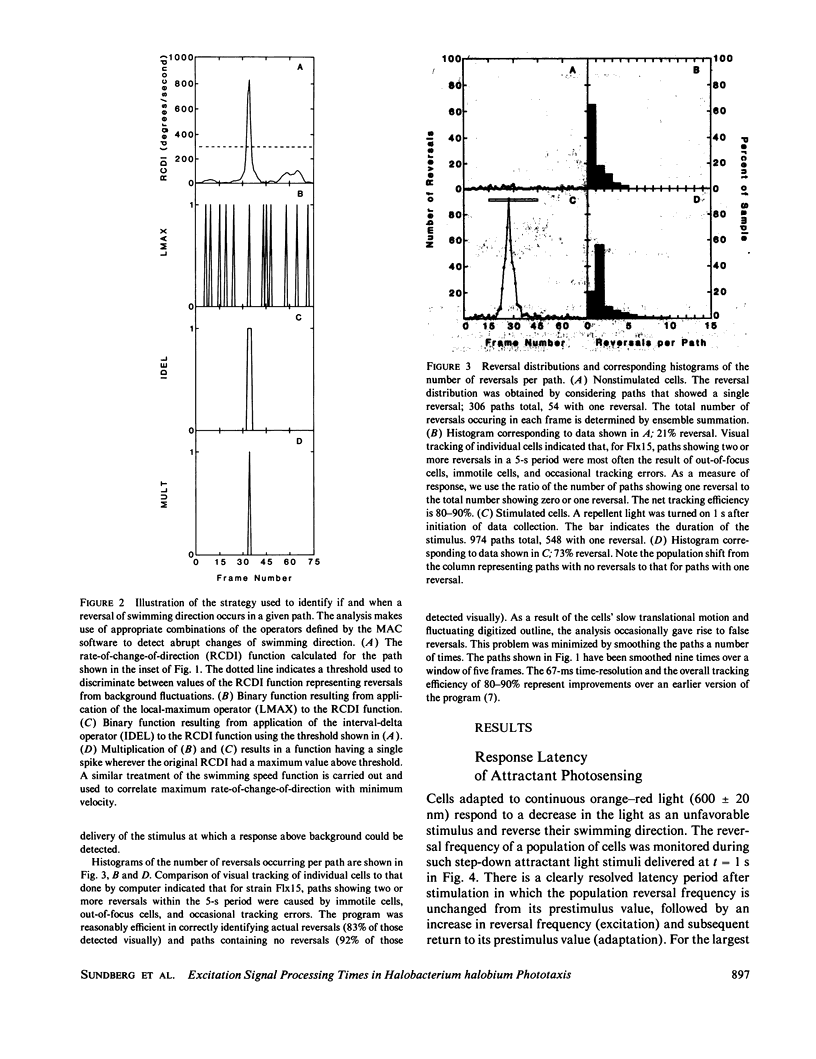
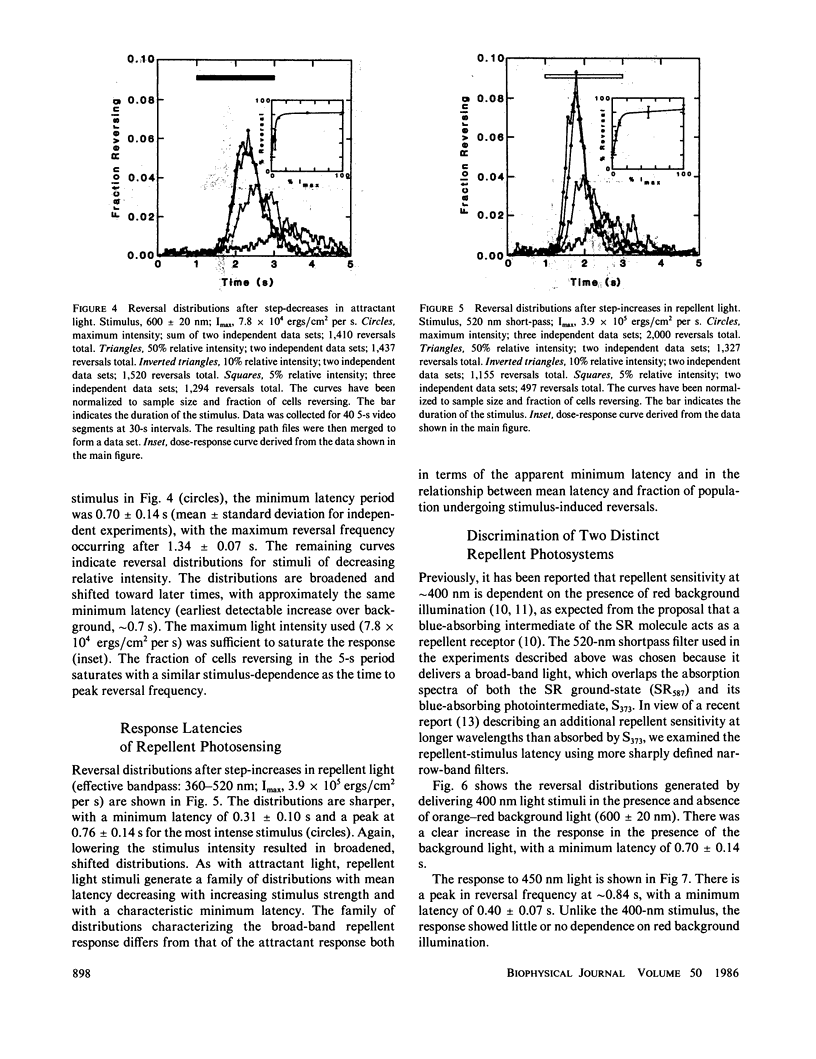
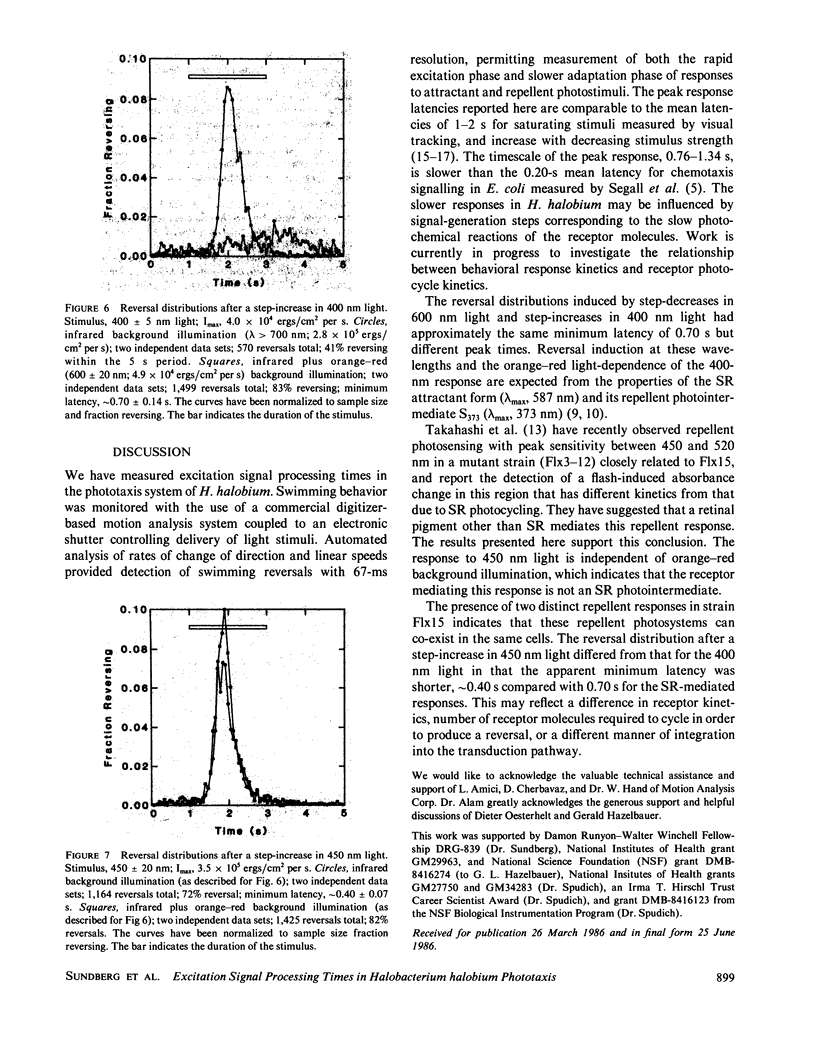
Phototaxis responses of Halobacterium halobium were monitored with a computerized cell-tracking system coupled to an electronic shutter controlling delivery of photostimuli. Automated analysis of rates of change in direction and linear speeds provided detection of swimming reversals with 67 ms resolution, permitting measurement of distinct phases of the responses to attractant and repellent stimuli. After stimulation, there was a latency period in which the population reversal frequency was unchanged, followed by an excitation phase in which reversal frequency increased, and a slower adaptation phase in which reversal frequency returned to its prestimulus value. A step-decrease in illumination of the attractant receptor slow-cycling or sensory rhodopsin (SR) (lambda max, 587 nm) was interpreted by the cells as an unfavorable stimulus and, after a minimum latency of 0.70 +/- 0.14 s, induced swimming reversals with the peak response occurring 1.34 +/- 0.07 s after onset of the stimulus. Two distinct repellent responses in the near UV/blue were observed. One was a reversal response to 400 nm light, which was dependent on orange-red background illumination as expected for the photointermediate repellent form of SR (lambda max, 373 nm). The minimum latency of this response was approximately the same as that of the SR attractant system. The second was a reversal response with shorter minimum latency (0.40 +/- 0.07 s) to light of longer wavelength (450 nm) than absorbed by the known SR repellent form. This result confirms recent findings of an additional repellent photosystem in this spectral range. Further, the longer wavelength repellent response is independent of orange-red background illumination, indicating that the photoreceptor mediating this response is not a photointermediate of SR.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. Chemotaxis in bacteria. Annu Rev Biochem. 1975;44:341–356. doi: 10.1146/annurev.bi.44.070175.002013. [DOI] [PubMed] [Google Scholar]
- Bogomolni R. A., Spudich J. L. Identification of a third rhodopsin-like pigment in phototactic Halobacterium halobium. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6250–6254. doi: 10.1073/pnas.79.20.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dencher N. A., Hildebrand E. Sensory transduction in Halobacterium halobium: retinal protein pigment controls UV-induced behavioral response. Z Naturforsch C. 1979 Sep-Oct;34(9-10):841–847. doi: 10.1515/znc-1979-9-1030. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr Biochemistry of sensing and adaptation in a simple bacterial system. Annu Rev Biochem. 1981;50:765–782. doi: 10.1146/annurev.bi.50.070181.004001. [DOI] [PubMed] [Google Scholar]
- Macnab R. M., Koshland D. E., Jr The gradient-sensing mechanism in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2509–2512. doi: 10.1073/pnas.69.9.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segall J. E., Manson M. D., Berg H. C. Signal processing times in bacterial chemotaxis. Nature. 1982 Apr 29;296(5860):855–857. doi: 10.1038/296855a0. [DOI] [PubMed] [Google Scholar]
- Spudich E. N., Spudich J. L. Control of transmembrane ion fluxes to select halorhodopsin-deficient and other energy-transduction mutants of Halobacterium halobium. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4308–4312. doi: 10.1073/pnas.79.14.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. L., Bogomolni R. A. Mechanism of colour discrimination by a bacterial sensory rhodopsin. Nature. 1984 Dec 6;312(5994):509–513. doi: 10.1038/312509a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg S. A., Bogomolni R. A., Spudich J. L. Selection and properties of phototaxis-deficient mutants of Halobacterium halobium. J Bacteriol. 1985 Oct;164(1):282–287. doi: 10.1128/jb.164.1.282-287.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Mochizuki Y., Kamo N., Kobatake Y. Evidence that the long-lifetime photointermediate of s-rhodopsin is a receptor for negative phototaxis in Halobacterium halobium. Biochem Biophys Res Commun. 1985 Feb 28;127(1):99–105. doi: 10.1016/s0006-291x(85)80131-5. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Watanabe M., Kamo N., Kobatake Y. Negative phototaxis from blue light and the role of third rhodopsinlike pigment in halobacterium cutirubrum. Biophys J. 1985 Aug;48(2):235–240. doi: 10.1016/S0006-3495(85)83776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


