Abstract
It is widely believed that subunit vaccines composed of multiple components will offer greater protection against challenge by malaria, and yet there is little experimental evidence to support this view. We set out to test this proposition in the Plasmodium yoelii challenge system in rodents by comparing the degree of protection conferred by immunization with a mixture of merozoite surface proteins to that conferred by single proteins. We therefore examined a defined protein mixture made of the epidermal growth factor-like domains of P. yoelli merozoite surface protein 1 (MSP1) and MSP4/5, the homologue of P. falciparum MSP4 and MSP5. In the present study we demonstrate that this combination of recombinant proteins dramatically enhances protection against lethal malaria challenge compared to either protein administered alone. Many mice immunized with the MSP4/5 plus MSP119 combination did not develop detectable parasitemia after challenge. Combined immunization with MSP119 and yMSP4/5, a product characterized by lower protective efficacy, also greatly enhanced protection by reducing peak parasitemias and increasing the numbers of survivors. In some combination trials, levels of antibodies to MSP119 were elevated compared to the MSP119 alone group; however, improved protection occurred regardless of whether boosting of the anti-MSP119 response was observed. Boosting of anti-MSP119 did not appear to be due to contaminating endotoxin in the EcMSP4/5 material since enhanced protection was observed in C3H/HeJ mice, which are endotoxin insensitive. Collectively, these experiments show that multiantigen combinations offer enhanced levels of protection against asexual stage infection and suggest that combinations of MSP1, MSP4, and MSP5 should be evaluated further for use in humans.
The development of an effective vaccine against infection by malaria asexual stages faces numerous obstacles, including antigenic diversity and antigenic variation on the part of the parasite, lack of a reliable delivery system, and potent adjuvants approved for clinical use, as well as difficulties with large-scale production of clinical-grade vaccine antigen (10). Despite the significant progress that has been made in the identification of vaccine candidates, an effective vaccine against human malaria has not yet been developed. Antigen selection and characterization has been hampered by the lack of a readily available challenge system for Plasmodium falciparum. Consequently, rodent models for malaria have attracted much attention and proved to be useful in assessing antigenicity and immunogenicity of vaccine candidates. It is generally accepted that the eventual blood-stage vaccine will be composed of multiple antigens and will need to induce multiple immune responses in vaccinated individuals. The inclusion of multiple antigens in a vaccine may act synergistically on the host immune system, leading to an increase in antibody production or T-cell activation and enhanced protection.
The first multicomponent blood-stage vaccine to be tested in monkeys and then humans was SPf66 (26, 27). Other multicomponent and multistage vaccines include NYVAC-Pf7 (38), which consists of seven P. falciparum genes inserted into a highly attenuated vaccinia virus, and CDC/NIIMALVAC-1 (34), composed of a synthetic gene that encodes numerous B- and T-cell epitopes, as well as cytotoxic-T-lymphocyte epitopes derived from nine P. falciparum antigens. Another approach to the multivalent vaccine is immunization with a combination of recombinant proteins. A combination of different recombinant malaria antigens has been tested in humans. The vaccine consisted of MSP1, MSP2, and RESA (19), and although antibodies were induced against all three antigens, they did not have any significant effect on parasite growth after challenge compared to a placebo group. However, use of this combination in a field trial resulted in modest levels of protection (B. Genton, unpublished data). Despite the testing of a number of multiple-component formulations, convincing proof of greater efficacy for such combinations versus single-antigen formulations is not available.
We describe here immunization experiments with a combination of the defined Plasmodium yoelii merozoite surface protein 4/5 (MSP4/5) and MSP119. The results demonstrate that immunization with a combination of recombinant MSP4/5 and MSP119 induces enhanced protection in mice compared to protection observed after immunization with a single antigen. The groups of mice immunized with both antigens showed higher survival rates and significantly lower peak parasitemias compared to mice immunized with either of the two antigens alone. Patent parasitemia could not be detected in the majority of animals vaccinated with a combination of antigens, and the same outcome was obtained in mice with different genetic backgrounds.
MATERIALS AND METHODS
Parasites and animals.
P. yoelii YM parasites were kindly supplied by Michael F. Good (Queensland Institute of Medical Research, Brisbane, Queensland, Australia). Female BALB/c and C3H/HeJ mice, aged 6 to 8 weeks, were purchased from the Central Animal Services (Monash University, Melbourne, Victoria, Australia).
Recombinant proteins, expression, and purification.
The full-length PyMSP4/5 sequence lacking the predicted signal peptide and glycosylphosphatidylinositol (GPI) anchor, was expressed as a His6-tagged recombinant protein (EcMSP4/5) and purified on Talon metal affinity resin (Clontech, Palo Alto, Calif.) as described previously (14). The full-length yMSP4/5 (lacking signal sequence and GPI attachment site) and yMSP119 were expressed in Saccharomyces cerevisiae as described previously (13).
Vaccination and challenge infection.
Groups of female BALB/c or C3H/HeJ mice were immunized with either 25 or 30 μg of various recombinant proteins emulsified in complete Freund adjuvant (Difco Laboratories, Detroit, Mich.) administered intraperitoneally (i.p.). Recombinant proteins for combination immunization were mixed in solution prior to coformulation in adjuvant. Two subsequent boosters of antigen emulsified in incomplete Freund adjuvant (Difco Laboratories) were delivered i.p. at monthly intervals. Control mice were injected with phosphate-buffered saline emulsified in the appropriate Freund adjuvant. Sera were collected prior to the initial injection and 2 days before challenge. At 12 to 14 days after the second booster, mice were challenged i.p. with 105 P. yoelii YM parasitized red blood cells. Parasitemias were monitored microscopically by Giemsa-stained thin blood smears fixed with methanol. Blood for smears was collected each day starting from day 3 and finishing at day 22 to 24 postinfection. A total of 500 cells per slide were counted.
Antibody assay.
Indirect enzyme-linked immunosorbent assays (ELISAs) were performed for antibody determination as previously described (15).
Statistics.
The Fisher exact probability test was used to determine the significance of differences in the number of surviving animals between the immunized and control groups. The Mann-Whitney test (U test) was used to determine the significance of differences in peak parasitemia and prechallenge antibody responses. GraphPad Prism software was used to perform all of the statistical analyses.
RESULTS
Combined immunization with MSP4/5 and MSP119.
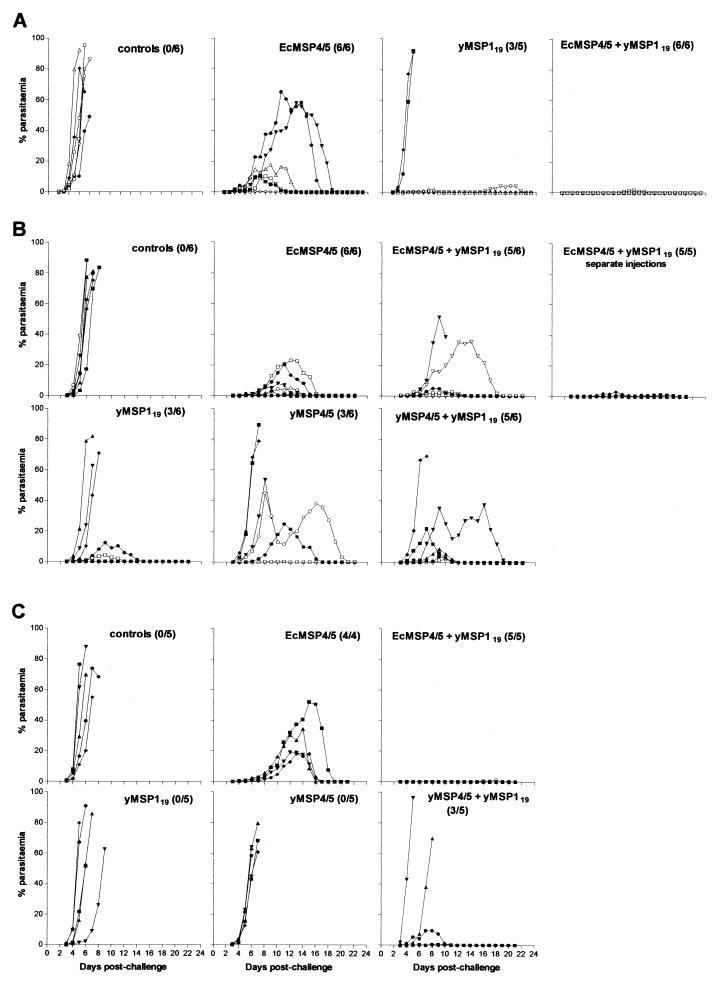
In three separate trials, groups of mice were immunized with a combination of either EcMSP4/5-yMSP119 or yMSP4/5-yMSP119. The appropriate doses of each antigen were administered in Freund adjuvant either mixed together or delivered as two separate injections. In trial 1, groups of mice were immunized with either 25 μg of EcMSP4/5 or 30 μg of yMSP119 or a combination of both antigens. Control mice were injected with phosphate-buffered saline in adjuvant, and all mice in this group succumbed to infection by day 9 postchallenge (Fig. 1A). In the group immunized with yMSP119 there were three mice out of five that survived, whereas in the EcMSP4/5 group all mice were able to control the infection. Immunization with a combination of proteins had a dramatic effect on the levels of protection. All of the six mice survived the challenge with five mice showing no detectable parasitemia (Fig. 1A). The only mouse that developed parasitemia had a peak of 1.6%, and the infection was patent for 6 days. The differences in peak parasitemias between different groups were statistically significant (Mann-Whitney test) with a P value of 0.004 when the comparison was made between EcMSP4/5 and the combined antigen groups and 0.03 when comparison was made between yMSP119 and the combined antigen groups. In trials 2 (Fig. 1B) and 3 (Fig. 1C), mice were immunized with 25 μg of either EcMSP4/5, yMSP119, yMSP4/5, or a combination of yMSP119 with either of the MSP4/5 preparations. The numbers of survivors in groups of mice immunized with either preparation of MSP4/5 were similar to those observed in trial 1 and our previous studies (15, 16). Unexpectedly, all mice died in a group immunized with yMSP119 (trial 3), which is in contrast with our previous observations (trial 1 and 2) and those of others (11). When a combination of yeast-expressed antigens was used for immunization, an improvement in both the peak parasitemia and survival rates was observed compared to groups of mice immunized with separate antigens, particularly in trial 3. In groups of mice immunized with a combination of yMSP4/5 and yMSP119, 8 of 11 mice (in two separate trials) survived the challenge compared to 3 of 11 in the groups immunized with either antigen alone. The mean peak parasitemias were greatly reduced compared to the mean peak parasitemias observed in groups immunized with yMSP4/5 (23.8% versus 48.6% and 35.3% versus 66.2% in trials 2 and 3, respectively) and yMSP119 (23.8% versus 38.6% and 35.3% versus 74% in trials 2 and 3, respectively). However, the overall differences between peak parasitemias did not reach statistical significance between the groups. In the group immunized with a combination of EcMSP4/5+yMSP119 (trial 2), five of six mice were capable of controlling the infection. The survival rates were improved compared to the group immunized with yMSP119 alone (five of six versus three of six mice, respectively) but not compared to the EcMSP4/5 group (five of six versus six of six mice, respectively). The mean peak parasitemia was reduced compared to the yMSP119 group (15.7% versus 38.6%) but not compared to EcMSP4/5 group (15.7% versus 10.1%). Differences in peak parasitemias between different groups were not statistically significant. However, two mice in the combined EcMSP4/5+yMSP119 group developed subpatent parasitemia, a phenomenon not observed in the group of mice immunized with EcMSP4/5 alone. In trial 3, the outcome of combined immunization was identical to that observed in trial 1. Four mice showed subpatent parasitemia, and one had a peak parasitemia of 1.2%. This was a dramatic improvement compared to the group immunized with yMSP119 (zero of five survivors) or EcMSP4/5 (1.2% versus 31% average peak parasitemia). The differences in peak parasitemias were statistically significant and resulted in a P value of 0.016 when the comparison was made between groups immunized with EcMSP4/5 and the combination of antigens, and it was 0.008 when the groups immunized with yMSP119 were compared with the groups given the combination of antigens.
FIG. 1.
Blood-stage parasitemias in groups of mice in trials 1, 2, and 3 (A, B, and C, respectively). The number of survivors out of the total number of mice is shown in brackets next to the title of each graph.
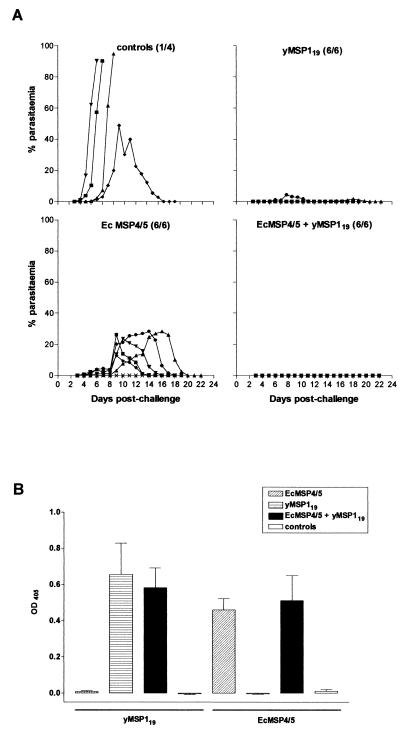
FIG. 3.
(A) Blood-stage parasitemia in groups of C3H/HeJ mice controls and immunized with 25 μg of either EcMSP4/5, yMSP119, or a combination of both administered as one injection. The number of survivors out of the total number of mice is shown in brackets next to the title of each graph. (B) Prechallenge antibody responses of C3H/HeJ mice cross-checked on yMSP119 and EcMSP4/5. Mean values of measurements of the optical density at 405 nm obtained from a 1:50,000 dilution (in duplicates) of individual sera from all mice in each group were plotted. Error bars represent the standard error of the mean.
An additional group of mice was immunized with 25 μg of yMSP119, followed by 25 μg of EcMSP4/5 emulsified in Freund adjuvant and delivered as two separate i.p. injections at a 2-day interval. All mice immunized with the combined antigens survived the challenge; however, all developed patent parasitemia (Fig. 1B). Nevertheless, the observed protection was greatly enhanced compared to any of the immunization trials conducted with a single antigen during the present study. The peak parasitemias ranged from 0.4 to 1.6%, and parasitemias were patent for a period of 4 to 7 days (Fig. 1B). There was a statistically significant difference in peak parasitemias between group of mice immunized with a combination of antigens administered by separate injections and the EcMSP4/5-immunized group (P = 0.03) and the yMSP119-immunized group (P = 0.08). There was no significant difference in peak parasitemias between groups of mice immunized with the combination of antigens administered either together or separately (trial 2).
ELISA analysis of prechallenge antibody responses.
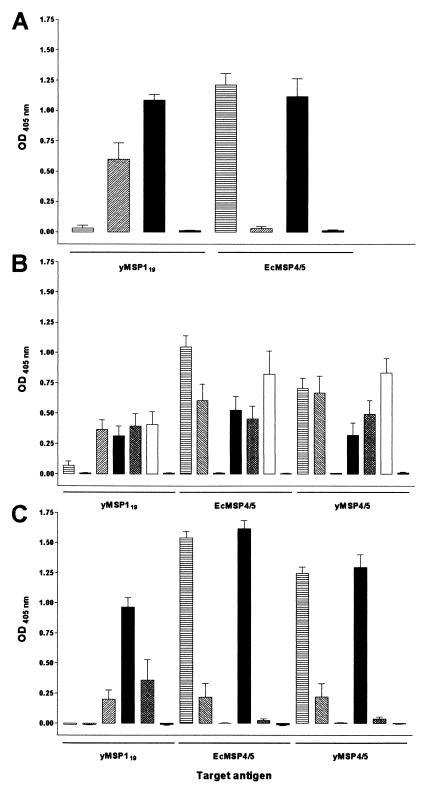
The prechallenge antibody responses were analyzed by ELISA on both of the antigens used for immunization (Fig. 2). In trial 1 (Fig. 2A) there was no difference in the prechallenge antibody responses between the combined antigen and EcMSP4/5-immunized groups when read out on target antigen EcMSP4/5. However, there was a significant difference (P = 0.008) between the combined antigen- and yMSP119-immunized groups, with the responses to yMSP119 being significantly boosted in the combined group. In trial 2, the prechallenge antibody responses were read out on the three different antigens: EcMSP4/5, yMSP4/5, and yMSP119 (Fig. 2B). There was no increase in anti-yMSP119 prechallenge antibody responses in the combined group compared to the yMSP119-immunized group. Statistically significant differences were only observed between the combined immunization group (EcMSP4/5+yMSP119, one injection) and the EcMSP4/5 group when read out on EcMSP4/5 (P = 0.015) and between the combined groups (EcMSP4/5+yMSP119) administered by either one or two injections when read out on yMSP4/5 (P = 0.009). Differences in prechallenge antibody responses between other groups of mice immunized with the combination of antigens administered together or separately between groups of mice immunized with either of the antigens alone and the group immunized with the combination of antigens administered separately did not reach statistical significance. In trial 3 (Fig. 2C) there was no difference in prechallenge antibody responses between the EcMSP4/5+yMSP119 combination and EcMSP4/5-immunized groups. On the other hand, anti-MSP1 prechallenge responses were again significantly boosted (P = 0.008) in the combined immunization group compared to yMSP119-immunized animals when read out on yMSP119. Interestingly, prechallenge antibody responses to yMSP4/5 in a group immunized with a combination of two yeast-derived malarial antigens were remarkably low (lower than those in the yMSP4/5-immunized group). When cross-checked on yMSP119, these responses were higher than those in the yMSP119-immunized group, but the difference was not statistically significant. We have also investigated the isotype distribution in mice immunized with a combination of antigens (trial 1). The isotype profile in these mice was similar to that observed in our previous studies (15) and immunoglobulin G2a was the dominant immunoglobulin type. The isotype was identical when read out on either target antigen (data not shown).
FIG. 2.
Prechallenge antibody responses of mice in trials 1, 2, and 3 (A, B, and C, respectively) cross-checked on yMSP119, EcMSP4/5, and yMSP4/5. Mean values of optical density at 405 nm are shown; measurements obtained from a 1:5,000 dilution (in duplicates) of individual sera from all mice in each group were plotted. Error bars represent the standard error of the mean. Bars: ▤, EcMSP4/5; ▧, yMSP4/5; ▨, yMSP119; ▪, yMSP119 + EcMSP4/5; ▩, yMSP119 + yMSP4/5; □, yMSP119 + EcMSP4/5 (separate injections); ░⃞, controls.
Immunization of endotoxin nonresponding C3H/HeJ mice.
In order to investigate the possible mechanisms behind the boosting of anti-MSP1 titers after combined immunization and to test whether there is a correlation between increased anti-MSP1 titers and enhanced protection observed in mice immunized with a combination of antigens, we performed an immunization trial in C3H/HeJ mice. These mice do not respond normally to endotoxin due to a mutation in Tlr4 gene selectively impeding lipopolysaccharide (LPS) signal transduction (29, 30). Groups of four to six mice were immunized as described in Materials and Methods section with 25 μg of either antigen or a combination of both administered as one injection. Three of four control mice succumbed to infection, whereas one was able to fully recover with a peak parasitemia of 49%, which was significantly higher than peak parasitemias in any other group (Fig. 3A). All immunized mice survived the challenge infection. Mice immunized with EcMSP4/5 had a mean peak parasitemia of 19.8%, and the course of infection was identical to that observed in the present study (Fig. 1) or described previously (15). There was one mouse that showed no detectable parasitemia after P. yoelii challenge, a phenomenon not observed before in BALB/c mice immunized with EcMSP4/5 alone. Only two of six mice immunized with yMSP119 had patent parasitemia with peaks of 1.8 and 4.4%. The remaining four mice had no patent parasitemia. All mice immunized with a combination of both antigens were resistant to infection. ELISA analysis of the prechallenge immune sera that were compared on both EcMSP4/5 and yMSP119 showed no boosting of anti-MSP1 titers in the group immunized with a combination of antigens. In fact, there was no significant difference in prechallenge antibody responses between the group immunized with a combination of EcMSP4/5 and yMSP119 and any of the groups immunized by either antigen alone (Fig. 3B).
DISCUSSION
A wealth of published reports supports the notion that immunization with a single antigen can confer protection to immunized animals, and this has been clearly demonstrated for the major malaria vaccine candidates AMA1 (1, 5) and MSP1 (7, 11, 12, 20). We have previously shown that immunization with recombinant MSP4/5 can induce protection against lethal challenge with P. yoelii YM (15) and that the degree of the induced protection was affected by the form of the antigen (16). In the majority of immunization trials using rodent malaria systems, immunized mice developed patent parasitemia despite being able to control the infection and fully recover after challenge. Immunization with MSP119 by a prolonged regimen with combinations of subcutaneous and i.p. injection was able to induce sterile protection in vaccinated mice (11). However, the MSP1 immunization trials performed in the present study showed that, after immunization, mice either developed very good protection (low peak or subpatent parasitemia) or succumbed to infection with fulminating parasitemia similar to that observed in control mice. In contrast, immunization with recombinant MSP4/5 usually results in 100% survival of immunized animals; however, a fraction of immunized mice develops high peak parasitemias. Neither of these results would be acceptable as outcomes in human clinical trials.
The usual solution suggested to this problem is that of a multicomponent vaccine containing proteins from one or several life cycle stages of the parasite. However, there is little experimental evidence to suggest that this would work for malaria. Trials with antigen combinations such as Spf66 and NYVAC-7 have given inconsistent or poor results (3, 23, 24, 35, 39), and in none of these experiments is there evidence that the combination performs better than single antigens. Shi et al. (34) demonstrated the ability to raise growth-inhibitory sera in rabbits by immunization with multiple blood-stage epitopes but did not demonstrate that this approach was more effective than a particular epitope given singly. Immunization with a combination of a region of MSP1, RESA, and MSP2 in humans gave rise to low antibody responses and little evidence of subsequent protection in a small group of naive individuals (19, 33). The combination of a second antigen, TRAP, with CSP led to a failure to induce the level of protection noted with CSP alone (17, 18, 36). On the other hand, Wang et al. (41) have shown that mice can be protected against a sporozoite challenge by immunization with a combination of CSP and MSP1. Although the levels of induced antibodies to antigens delivered in combination were markedly reduced, an additive effect of such immunization was observed in 52% of mice. The lessons from vaccines in other parasites are not clearcut either. For example, in the Fasciola hepatica system, the combination of the two antigens cathepsin L proteinase and hemoglobin led to augmented immunity to challenge (6). However, the degree of immunity induced by leucine aminopeptidase was significantly decreased when it was combined with two cathepsin L proteinases, which on their own were moderately protective (28). In studies on Schistosoma vaccines, many candidate antigens have been identified, but in the limited data set on combinations of antigens there is little compelling data for enhanced efficacy (37).
The present study therefore provides the first compelling evidence for markedly improved protection against malaria by protein combinations in an asexual blood-stage vaccine. In all cases, the degree of immunity was enhanced by using antigen combinations, although the absolute level of protection varied between trials. Thus, immunity was strongest when Escherichia coli-derived MSP4/5 was used in combination with MSP119, but a clear additive effect occurred even when both antigens were produced in a yeast expression system. This agrees with previous studies that have shown that EcMSP4/5 induces higher levels of protection than MSP4/5 produced in a yeast expression system although the reason for this is still not known (16). The protective efficacy of MSP4/5 immunization is correlated with the level of antibody induced (15), and we found the same dependence on induced antibody levels with anti-MSP119 responses, which suggests that antibody is clearly important (9, 11, 25). The antibody responses induced by the two proteins are not cross-reactive (data not shown) and react with separate proteins on the merozoite surface. The most likely explanation for enhanced protection is therefore that, collectively, the two sets of binding antibodies more effectively coat the merozoite surface and interfere with invasion while making the merozoite a better target for ADCI (4).
The particular experimental design chosen was to examine the effect of combining antigen doses such that mice received 50 μg of total protein compared to 25 μg in either group given a single antigen. An alternate protocol would have been to match the total dose of the group receiving the combined antigens to that received by the single-antigen group; therefore, each mouse would receive 12.5 μg of MSP4/5 and 12.5 μg of MSP119. We felt it would be of interest to use the minimum dose that had been previously shown to give substantial levels of protection. We could then determine whether the protection could be substantially enhanced, since parasitemia levels commonly achieved in rodent system trials are still much higher than would be acceptable in humans. Of the mice receiving EcMSP4/5 and yMSP119, 64% had undetectable levels of parasitemia. The method of parasitemia determination used has a lower limit of detection of 0.2%, and such levels approach those that would be an acceptable outcome in human trials.
An interesting phenomenon encountered in some of the trials is that of boosting the response to MSP119 administered in combination with MSP4/5 compared to that seen when MSP119 is administered by itself. In the trials where this occurred such as trial 1, it is possible that enhanced protection is partially due to the boosting of MSP119 responses; however, enhanced protection occurred independent of this phenomenon as, for example, in trials 2 and 3 and in C3H/HeJ mice. The reason for the occasional anti-MSP119 boosting during combined immunization is unclear. It may be due to LPS contamination of the EcMSP4/5 preparation, since boosting did not occur when yeast-derived material was used or when LPS-insensitive mice were immunized. This hypothetical “LPS boosting” effect would have to be able to occur in mice immunized with complete Freund adjuvant, and this seems somewhat surprising. Furthermore, in mice immunized with the two antigens given on different days, boosting of the anti-MSP119 response did not occur. This may suggest that boosting requires some form of protein association, and it is possible that the two proteins may combine to form multimers via their epidermal growth factor-like domains, resulting in an aggregated form that is more immunogenic than the proteins given alone. Although there is no experimental evidence to support this theory in the present study, it is known that proteins with epidermal growth factor-like domains are involved in protein-protein interactions (8). However, in such a case it is not clear why there is no concomitant boost in the anti-MSP4/5 response. Whatever the mechanism for this boosting, it is clearly not required to induce synergistic protection. The main conclusion of these experiments is that the antigen combination tested is always more efficacious than the separate antigens.
Would equivalent efficacy be obtainable simply by improving the immunogenicity of MSP119 as a single antigen? Previous studies have shown that the dose used in the trial induces optimal levels of antibody (7), and there is no evidence to suggest that increased doses of MSP119 would reliably induce substantial increase in the antibody response. Increases in antibody levels are achievable by extended immunization protocols (11), but such protocols, requiring at least five doses of antigen administered in multiple sites, are unlikely to be applicable in humans. The most important factor favoring antigen combinations, however, is the observation that MSP119, and AMA1, for that matter, do not protect against challenge with heterologous parasite strain (5, 31, 32). The addition of a second antigen, particularly one that is relatively invariant, is likely to help overcome failure of protection against heterologous challenge.
How general is the phenomenon of enhanced protection by antigen combination? Clearly, particular antigen combinations need to be validated by experiment. In the absence of a vaccine of known efficacy for comparative testing, the predictive value of the rodent malaria model, particularly for evaluating combinations, is not known. For some antigens such as MSP2, testing in the rodent system will not be possible since a rodent malaria homologue of MSP2 apparently does not exist (2). Nevertheless, the leading vaccine candidates for asexual stage vaccines in humans such as AMA1 and MSP119 have shown excellent efficacy in rodent challenge systems (1, 5, 11), and primate systems have not yet been shown to be superior.
On the basis of the experiments reported here, we would suggest that the efficacy of MSP119 in human trials could be enhanced by combining it with the P. falciparum MSP4/5 homologues. There are two distinct homologues, MSP4 and MSP5, and there is no available evidence to suggest that one or the other is likely to be more important for the induction of protective immunity in humans. Indeed, since they have arisen by ancient gene duplication, it seems reasonable to suggest that they have evolved to provide related but distinct functions in the parasite, such as involvement in alternative invasion pathways. Their small size and relative invariance in sequence makes them attractive candidates for the production by recombinant DNA technology (21, 22, 40, 42). At present we suggest that both proteins (MSP4 and MSP5) should be included and tested in clinical trials in combination with MSP119. Additional immunization experiments in the rodent malaria system with combinations, including other blood-stage vaccine candidates, such as AMA1 or MSP8, together with MSP4/5 and MSP1, may show improved anti-parasite protection and are worthy of further study.
Acknowledgments
We thank David Kaslow and Louis Miller for assistance in manuscript preparation.
This work was supported by the National Health and Medical Research Council, the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases, and the Howard Hughes Medical Institute International Scholars in Parasitology and Infectious Diseases Program. L.K. and M.W.G. are recipients of Australian Postgraduate Award Scholarships.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Anders, R. F., P. E. Crewther, S. Edwards, M. Margetts, M. L. Matthew, B. Pollock, and D. Pye. 1998. Immunization with recombinant AMA-1 protects mice against infection with Plasmodium chabaudi. Vaccine 16:240-247. [DOI] [PubMed] [Google Scholar]
- 2.Black, C. G., L. Wang, A. R. Hibbs, E. Werner, and R. L. Coppel. 1999. Identification of the Plasmodium chabaudi homologue of merozoite surface proteins 4 and 5 of Plasmodium falciparum. Infect. Immun. 67:2075-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bojang, K. A., S. K. Obaro, U. D'Alessandro, S. Bennett, P. Langerock, G. A. Targett, and B. M. Greenwood. 1998. An efficacy trial of the malaria vaccine SPf66 in Gambian infants: second year of follow-up. Vaccine 16:62-67. [DOI] [PubMed] [Google Scholar]
- 4.Bouharoun-Tayoun, H., C. Oeuvray, F. Lunel, and P. Druilhe. 1995. Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J. Exp. Med. 182:409-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crewther, P. E., M. L. S. M. Matthew, R. H. Flegg, and R. F. Anders. 1996. Protective immune responses to apical membrane antigen 1 of Plasmodium chabaudi involve recognition of strain-specific epitopes. Infect. Immun. 64:3310-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalton, J. P., S. McGonigle, T. P. Rolph, and S. J. Andrews. 1996. Induction of protective immunity in cattle against infection with Fasciola hepatica by vaccination with cathepsin L proteinases and with hemoglobin. Infect. Immun. 64:5066-5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daly, T. M., and C. A. Long. 1995. Humoral response to a carboxyl-terminal region of the merozoite surface protein-1 plays a predominant role in controlling blood-stage infection in rodent malaria. J. Immunol. 155:236-243. [PubMed] [Google Scholar]
- 8.Davis, C. G. 1990. The many faces of epidermal growth factor repeats. New Biol. 2:410-419. [PubMed] [Google Scholar]
- 9.Dodoo, D., T. G. Theander, J. A. Kurtzhals, K. Koram, E. Riley, B. D. Akanmori, F. K. Nkrumah, and L. Hviid. 1999. Levels of antibody to conserved parts of Plasmodium falciparum merozoite surface protein 1 in Ghanaian children are not associated with protection from clinical malaria. Infect. Immun. 67:2131-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Good, M. F., D. C. Kaslow, and L. H. Miller. 1998. Pathways and strategies for developing a malaria blood-stage vaccine. Annu. Rev. Immunol. 16:57-87. [DOI] [PubMed] [Google Scholar]
- 11.Hirunpetcharat, C., J. H. Tian, D. C. Kaslow, N. van Rooijen, S. Kumar, J. A. Berzofsky, L. H. Miller, and M. F. Good. 1997. Complete protective immunity induced in mice by immunization with the 19-kilodalton carboxyl-terminal fragment of the merozoite surface protein-1 (MSP119) of Plasmodium yoelii expressed in Saccharomyces cerevisiae: correlation of protection with antigen-specific antibody titer, but not with effector CD4+ T cells. J. Immunol. 159:3400-3411. [PubMed] [Google Scholar]
- 12.Holder, A. A., and R. R. Freeman. 1981. Immunization against blood-stage rodent malaria using purified parasite antigens. Nature 294:361-364. [DOI] [PubMed] [Google Scholar]
- 13.Kaslow, D. C., G. Hui, and S. Kumar. 1994. Expression and antigenicity of Plasmodium falciparum major merozoite surface protein (MSP119) variants secreted from Saccharomyces cerevisiae. Mol. Biochem. Parasitol. 63:283-289. [DOI] [PubMed] [Google Scholar]
- 14.Kedzierski, L., C. G. Black, and R. L. Coppel. 2000. Characterization of the merozoite surface protein 4/5 gene of Plasmodium berghei and Plasmodium yoelii. Mol. Biochem. Parasitol. 105:137-147. [DOI] [PubMed] [Google Scholar]
- 15.Kedzierski, L., C. G. Black, and R. L. Coppel. 2000. Immunization with recombinant Plasmodium yoelii merozoite surface protein 4/5 protects mice against lethal challenge. Infect. Immun. 68:6034-6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kedzierski, L., C. G. Black, A. W. Stowers, M. W. Goschnick, D. C. Kaslow, and R. L. Coppel. 2001. Comparison of the protective efficacy of yeast-derived and Escherichia coli-derived recombinant merozoite surface protein 4/5 against lethal challenge by Plasmodium yoelii. Vaccine 19:4661-4668. [DOI] [PubMed] [Google Scholar]
- 17.Kester, K. E., D. G. Heppner, C. F. Ockenhouse, B. T. Hall, G. Voss, E. de Buyl, M. Van Handenhove, D. M. Gordon, U. Krzych, M.-C. Dubois, M. Delchambre, N. Tornieporth, L. Vigneron, W. R. Ballou, and J. Cohen. 2000. Double blind, randomized Phase I/IIa trial of SmithKline Beecham's candidate malaria vaccine RTS,S+TRAP and TRAP, p. 34. Molecular Approaches to Malaria, Lorne, Victoria, Australia.
- 18.Kester, K. E., D. A. McKinney, N. Tornieporth, C. F. Ockenhouse, D. G. Heppner, T. Hall, U. Krzych, M. Delchambre, G. Voss, M. G. Dowler, J. Palensky, J. Wittes, J. Cohen, and W. R. Ballou. 2001. Efficacy of recombinant circumsporozoite protein vaccine regimens against experimental Plasmodium falciparum malaria. J. Infect. Dis. 183:640-647. [DOI] [PubMed] [Google Scholar]
- 19.Lawrence, G., Q. Cheng, C. Reed, D. Taylor, A. Stowers, N. Cloonan, C. Rzepczyk, A. Smillie, K. Anderson, D. Pombo, A. Allworth, D. Eisen, R. Anders, and A. Saul. 2000. Effect of vaccination with 3 recombinant asexual-stage malaria antigens on initial growth rates of Plasmodium falciparum in non-immune volunteers. Vaccine 18:1925-1931. [DOI] [PubMed] [Google Scholar]
- 20.Ling, I. T., S. A. Ogun, and A. A. Holder. 1994. Immunization against malaria with a recombinant protein. Parasite Immunol. 16:63-67. [DOI] [PubMed] [Google Scholar]
- 21.Marshall, V. M., A. Silva, M. Foley, S. Cranmer, L. Wang, D. J. McColl, D. J. Kemp, and R. L. Coppel. 1997. A second merozoite surface protein (MSP-4) of Plasmodium falciparum that contains an epidermal growth factor-like domain. Infect. Immun. 65:4460-4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marshall, V. M., T. Wu, and R. L. Coppel. 1998. Close linkage of three merozoite surface protein genes on chromosome 2 of Plasmodium falciparum. Mol. Biochem. Parasitol. 94:13-25. [DOI] [PubMed] [Google Scholar]
- 23.Masinde, G. L., D. J. Krogstad, D. M. Gordon, and P. E. Duffy. 1998. Immunization with SPf66 and subsequent infection with homologous and heterologous Plasmodium falciparum parasites. Am. J. Trop. Med. Hyg. 59:600-605. [DOI] [PubMed] [Google Scholar]
- 24.Ockenhouse, C. F., P. F. Sun, D. E. Lanar, B. T. Wellde, B. T. Hall, K. Kester, J. A. Stoute, A. Magill, U. Krzych, L. Farley, R. A. Wirtz, J. C. Sadoff, D. C. Kaslow, S. Kumar, L. W. Church, J. M. Crutcher, B. Wizel, S. Hoffman, A. Lalvani, A. V. Hill, J. A. Tine, K. P. Guito, C. de Taisne, R. Anders, T. Horii, E. Paoletti, and W. R. Ballou. 1998. Phase I/IIa safety, immunogenicity, and efficacy trial of NYVAC-Pf7, a pox-vectored, multiantigen, multistage vaccine candidate for Plasmodium falciparum malaria. J. Infect. Dis. 177:1664-1673. [DOI] [PubMed] [Google Scholar]
- 25.O'Donnell, R. A., T. F. de Koning-Ward, R. A. Burt, M. Bockarie, J. C. Reeder, A. F. Cowman, and B. S. Crabb. 2001. Antibodies against merozoite surface protein (MSP)-119 are a major component of the invasion-inhibitory response in individuals immune to malaria. J. Exp. Med. 193:1403-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patarroyo, M. E., R. Amador, P. Clavijo, A. Moreno, F. Guzman, P. Romero, R. Tascon, A. Franco, L. A. Murillo, G. Ponton, and G. Trujillo. 1988. A synthetic vaccine protects humans against challenge with asexual blood stages of Plasmodium falciparum malaria. Nature 332:158-161. [DOI] [PubMed] [Google Scholar]
- 27.Patarroyo, M. E., P. Romero, M. L. Torres, P. Clavijo, A. Moreno, A. Martinez, R. Rodriguez, F. Guzman, and E. Cabezas. 1987. Induction of protective immunity against experimental infection with malaria using synthetic peptides. Nature 328:629-632. [DOI] [PubMed] [Google Scholar]
- 28.Piacenza, L., D. Acosta, I. Basmadjian, J. P. Dalton, and C. Carmona. 1999. Vaccination with cathepsin L proteinases and with leucine aminopeptidase induces high levels of protection against fascioliasis in sheep. Infect. Immun. 67:1954-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 30.Qureshi, S. T., L. Lariviere, G. Leveque, S. Clermont, K. J. Moore, P. Gros, and D. Malo. 1999. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J. Exp. Med. 189:615-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Renia, L., I. T. Ling, M. Marussig, F. Miltgen, A. A. Holder, and D. Mazier. 1997. Immunization with a recombinant C-terminal fragment of Plasmodium yoelii merozoite surface protein 1 protects mice against homologous but not heterologous P. yoelii sporozoite challenge. Infect. Immun. 65:4419-4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rotman, H. L., T. M. Daly, and C. A. Long. 1999. Plasmodium: immunization with carboxyl-terminal regions of MSP-1 protects against homologous but not heterologous blood-stage parasite challenge. Exp. Parasitol. 91:78-85. [DOI] [PubMed] [Google Scholar]
- 33.Saul, A., G. Lawrence, A. Smillie, C. M. Rzepczyk, C. Reed, D. Taylor, K. Anderson, A. Stowers, R. Kemp, A. Allworth, R. F. Anders, G. V. Brown, D. Pye, P. Schoofs, D. O. Irving, S. L. Dyer, G. C. Woodrow, W. R. Briggs, R. Reber, and D. Sturchler. 1999. Human phase I vaccine trials of 3 recombinant asexual stage malaria antigens with Montanide ISA720 adjuvant. Vaccine 17:3145-3159. [DOI] [PubMed] [Google Scholar]
- 34.Shi, Y. P., S. E. Hasnain, J. B. Sacci, B. P. Holloway, H. Fujioka, N. Kumar, R. Wohlhueter, S. L. Hoffman, W. E. Collins, and A. A. Lal. 1999. Immunogenicity and in vitro protective efficacy of a recombinant multistage Plasmodium falciparum candidate vaccine. Proc. Natl. Acad. Sci. USA 96:1615-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stanley, S. L., Jr. 1998. Malaria vaccines: are seven antigens better than one? Lancet 352:1163-1164. [DOI] [PubMed] [Google Scholar]
- 36.Stoute, J. A., K. E. Kester, U. Krzych, B. T. Wellde, T. Hall, K. White, G. Glenn, C. F. Ockenhouse, N. Garcon, R. Schwenk, D. E. Lanar, P. Sun, P. Momin, R. A. Wirtz, C. Golenda, M. Slaoui, G. Wortmann, C. Holland, M. Dowler, J. Cohen, and W. R. Ballou. 1998. Long-term efficacy and immune responses following immunization with the RTS,S malaria vaccine. J. Infect. Dis. 178:1139-1144. [DOI] [PubMed] [Google Scholar]
- 37.Taylor, M. G., M. C. Huggins, F. Shi, J. Lin, E. Tian, P. Ye, W. Shen, C. G. Qian, B. F. Lin, and Q. D. Bickle. 1998. Production and testing of Schistosoma japonicum candidate vaccine antigens in the natural ovine host. Vaccine 16:1290-1298. [DOI] [PubMed] [Google Scholar]
- 38.Tine, J. A., D. E. Lanar, D. M. Smith, B. T. Wellde, P. Schultheiss, L. A. Ware, E. B. Kauffman, R. A. Wirtz, C. De Taisne, G. S. N. Hui, S. P. Chang, P. Church, M. R. Hollingdale, D. C. Kaslow, S. L. Hoffman, K. P. Guito, W. R. Ballou, J. C. Sadoff, and E. Paoletti. 1996. NYVAC-Pf7: a poxvirus-vectored, multiantigen, multistage vaccine candidate for Plasmodium falciparum malaria. Infect. Immun. 64:3833-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Urdaneta, M., A. Prata, C. J. Struchiner, C. E. Tosta, P. Tauil, and M. Boulos. 1998. Evaluation of SPf66 malaria vaccine efficacy in Brazil. Am. J. Trop. Med. Hyg. 58:378-385. [DOI] [PubMed] [Google Scholar]
- 40.Wang, L., V. M. Marshall, and R. L. Coppel. 2002. Limited polymorphism of the vaccine candidate merozoite surface protein 4 of Plasmodium falciparum. Mol. Biochem. Parasitol. 120:301-303. [DOI] [PubMed] [Google Scholar]
- 41.Wang, R., Y. Charoenvit, T. M. Daly, C. A. Long, G. Corradin, and S. L. Hoffman. 1996. Protective efficacy against malaria of a combination sporozoite and erythrocytic stage vaccine. Immunol. Lett. 53:83-93. [DOI] [PubMed] [Google Scholar]
- 42.Wu, T., C. G. Black, L. Wang, A. R. Hibbs, and R. L. Coppel. 1999. Lack of sequence diversity in the gene encoding merozoite surface protein 5 of Plasmodium falciparum. Mol. Biochem. Parasitol. 103:243-250. [DOI] [PubMed] [Google Scholar]