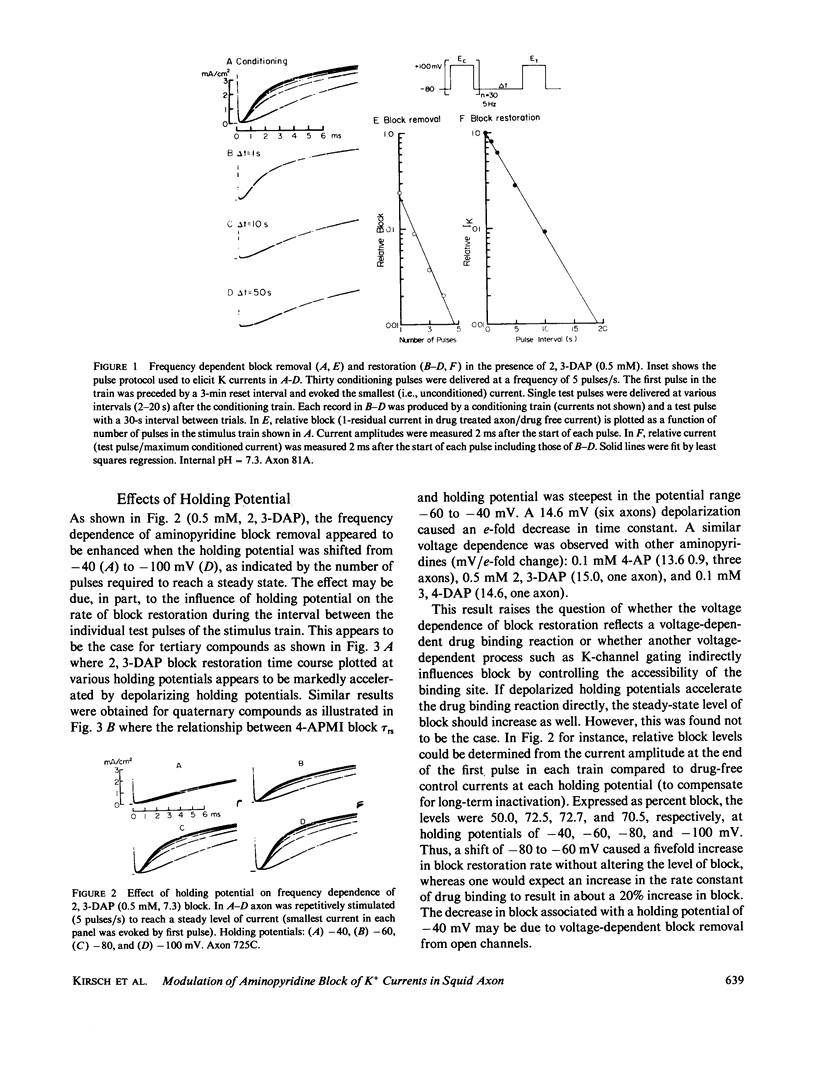
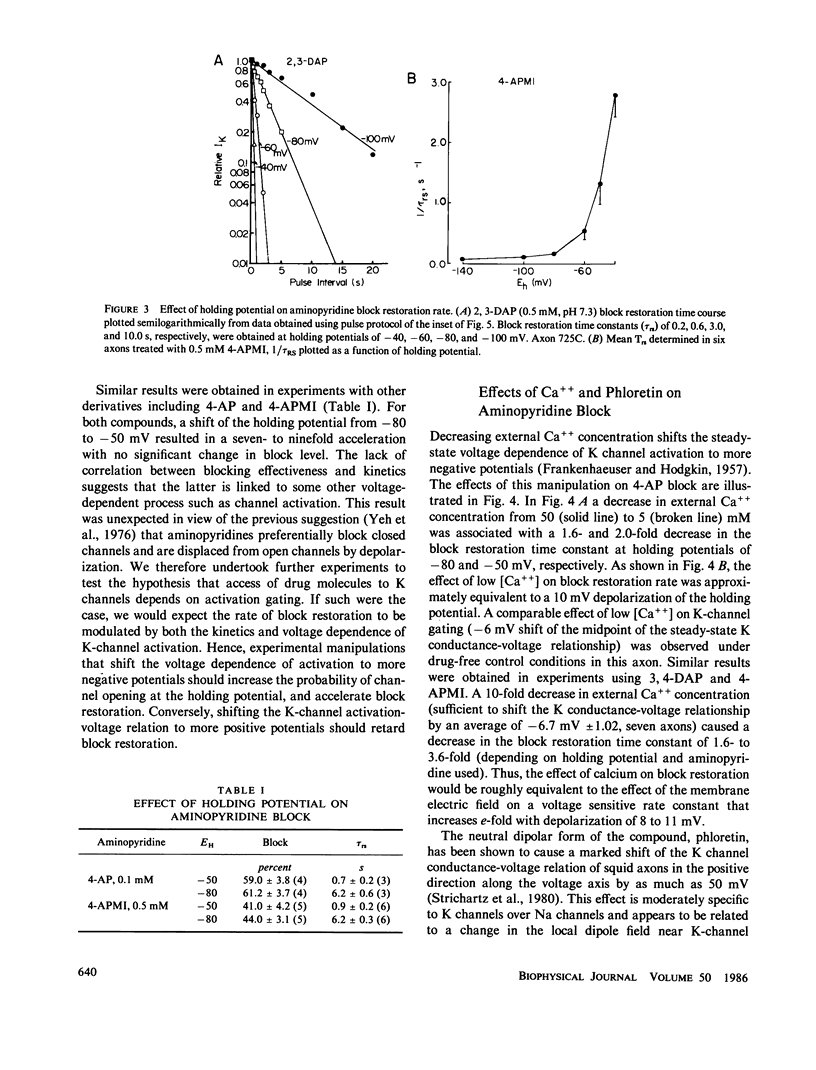
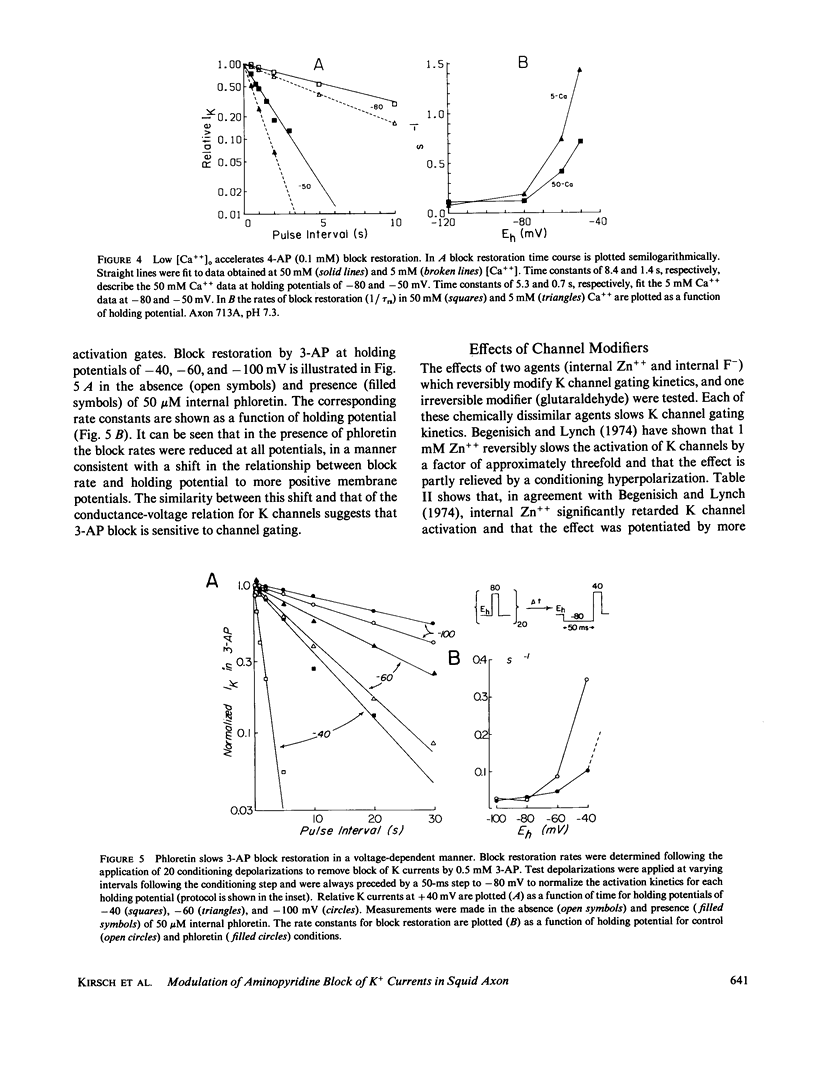
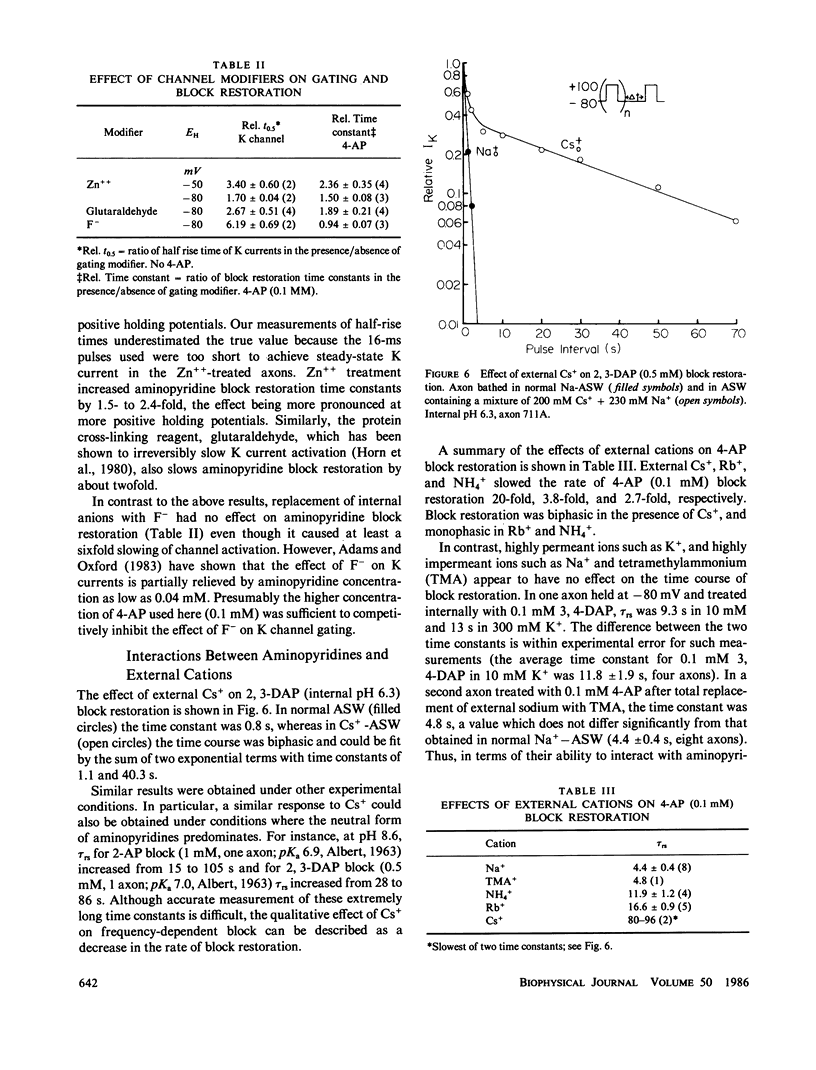
Abstract
Aminopyridines are known to block potassium (K) currents in excitable membranes in a manner dependent upon membrane potential, such that the block is relieved by depolarization and restored upon repolarization. In the present study, the effects of aminopyridines on voltage-dependent potassium (K) channels were examined in internally perfused, voltage-clamped squid giant axons. The time course of block restoration after conditioning depolarization was found to be modulated by membrane electric field, K-channel gating, and external cations. Depolarized holding potentials accelerated block restoration without altering steady-state block levels, suggesting that the voltage dependence of block restoration may be related to K channel gating rather than drug binding per se. In support of this notion, low external calcium concentration, which shifts the voltage dependence of K-channel gating to more negative potentials, also accelerated block restoration. Conversely, the relationship between the rate of block restoration and membrane holding potential was shifted in the depolarizing direction by phloretin, an agent that shifts the dependence of K-channel opening on membrane potential in a similar manner. Modification of K-channel gating also was found to alter the rate of block restoration. Addition of internal zinc or internal treatment with glutaraldehyde slowed the time course of both K-channel activation and aminopyridine block restoration. Aminopyridines also were found to interact in the K channel with external Cs+, NH4+, and Rb+, each of which slowed aminopyridine block restoration. Our results suggest that aminopyridines enter and occlude K channels, and that the availability of the binding site may be modulated by channel gating such that access is limited by the probability of the channel reaching an intermediate closed state at the resting potential.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Oxford G. S. Interaction of internal anions with potassium channels of the squid giant axon. J Gen Physiol. 1983 Oct;82(4):429–448. doi: 10.1085/jgp.82.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelman W. J., Jr, French R. J. Blocking of the squid axon potassium channel by external caesium ions. J Physiol. 1978 Mar;276:13–25. doi: 10.1113/jphysiol.1978.sp012217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W., Armstrong C. M. Survival of K+ permeability and gating currents in squid axons perfused with K+-free media. J Gen Physiol. 1980 Jan;75(1):61–78. doi: 10.1085/jgp.75.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAKER P. F., HODGKIN A. L., SHAW T. I. Replacement of the protoplasm of a giant nerve fibre with artificial solutions. Nature. 1961 Jun 3;190:885–887. doi: 10.1038/190885a0. [DOI] [PubMed] [Google Scholar]
- Begenisich T., Lynch C. Effects of internal divalent cations on voltage-clamped squid axons. J Gen Physiol. 1974 Jun;63(6):675–689. doi: 10.1085/jgp.63.6.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay J. R., Shlesinger M. F. Effects of external cesium and rubidium on outward potassium currents in squid axons. Biophys J. 1983 Apr;42(1):43–53. doi: 10.1016/S0006-3495(83)84367-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The after-effects of impulses in the giant nerve fibres of Loligo. J Physiol. 1956 Feb 28;131(2):341–376. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977 Apr;69(4):497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Potassium channels in myelinated nerve. Selective permeability to small cations. J Gen Physiol. 1973 Jun;61(6):669–686. doi: 10.1085/jgp.61.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B., Schwarz W. Potassium channels as multi-ion single-file pores. J Gen Physiol. 1978 Oct;72(4):409–442. doi: 10.1085/jgp.72.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Brodwick M. S., Eaton D. C. Effect of protein cross-linking reagents on membrane currents of squid axon. Am J Physiol. 1980 Mar;238(3):C127–C132. doi: 10.1152/ajpcell.1980.238.3.C127. [DOI] [PubMed] [Google Scholar]
- Kirsch G. E., Narahashi T. 3,4-diaminopyridine. A potent new potassium channel blocker. Biophys J. 1978 Jun;22(3):507–512. doi: 10.1016/S0006-3495(78)85503-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch G. E., Narahashi T. Site of action and active form of aminopyridines in squid axon membranes. J Pharmacol Exp Ther. 1983 Jul;226(1):174–179. [PubMed] [Google Scholar]
- Meves H., Pichon Y. The effect of internal and external 4-aminopyridine on the potassium currents in intracellularly perfused squid giant axons. J Physiol. 1977 Jun;268(2):511–532. doi: 10.1113/jphysiol.1977.sp011869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxford G. S. Some kinetic and steady-state properties of sodium channels after removal of inactivation. J Gen Physiol. 1981 Jan;77(1):1–22. doi: 10.1085/jgp.77.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strichartz G. R., Oxford G. S., Ramon F. Effects of the dipolar form of phloretin on potassium conductance in squid giant axons. Biophys J. 1980 Aug;31(2):229–246. doi: 10.1016/S0006-3495(80)85053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson R. P., Jr, Armstrong C. M. K+ channels close more slowly in the presence of external K+ and Rb+. Nature. 1981 Jun 4;291(5814):427–429. doi: 10.1038/291427a0. [DOI] [PubMed] [Google Scholar]
- White M. M., Bezanilla F. Activation of squid axon K+ channels. Ionic and gating current studies. J Gen Physiol. 1985 Apr;85(4):539–554. doi: 10.1085/jgp.85.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J. Z., Oxford G. S., Wu C. H., Narahashi T. Dynamics of aminopyridine block of potassium channels in squid axon membrane. J Gen Physiol. 1976 Nov;68(5):519–535. doi: 10.1085/jgp.68.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]


