Abstract
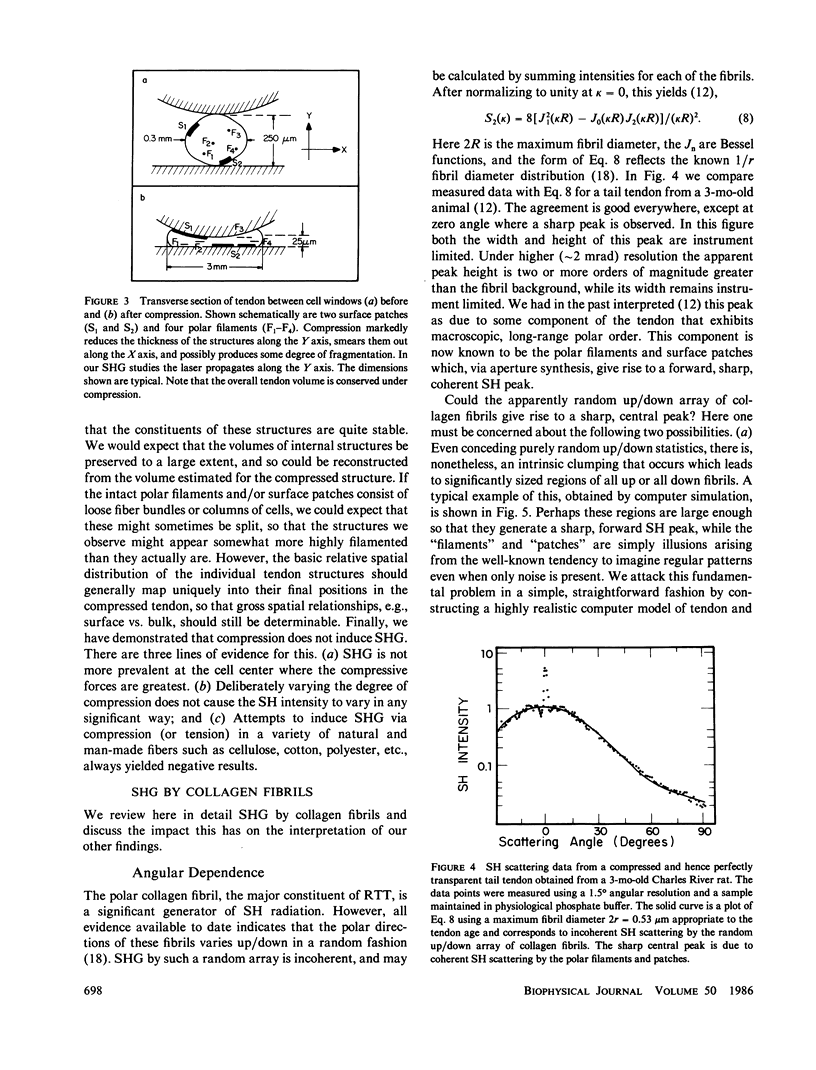
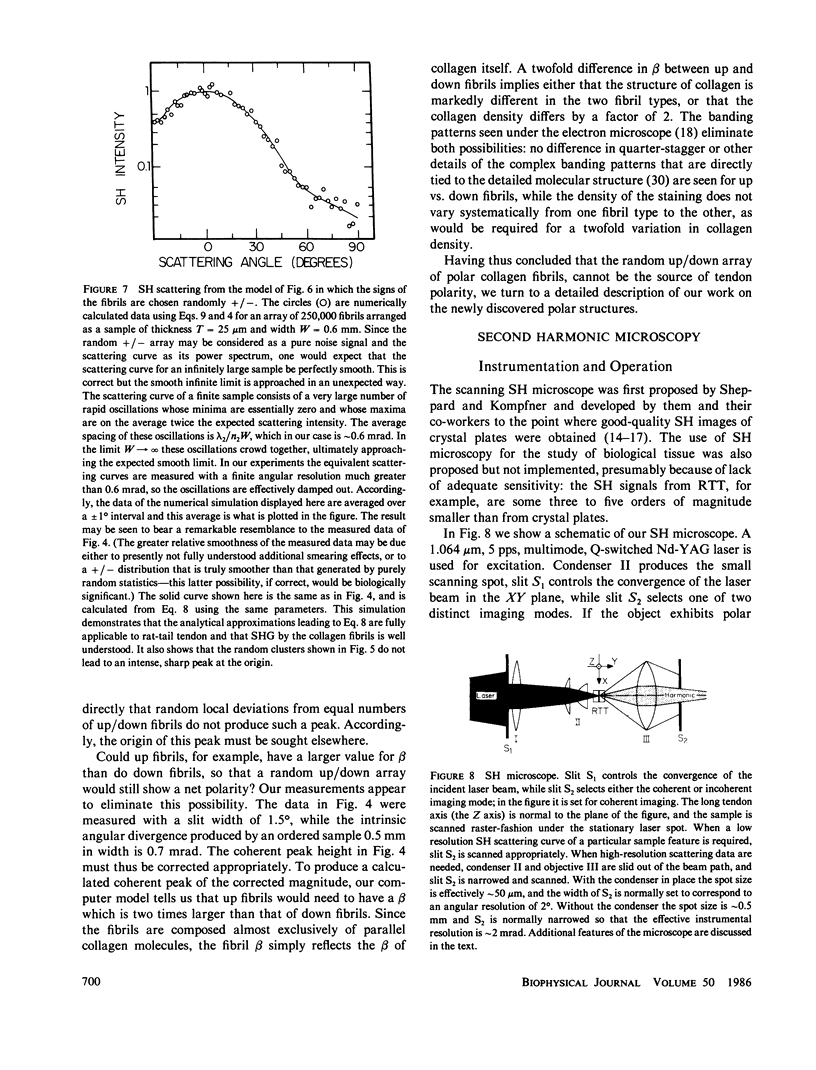
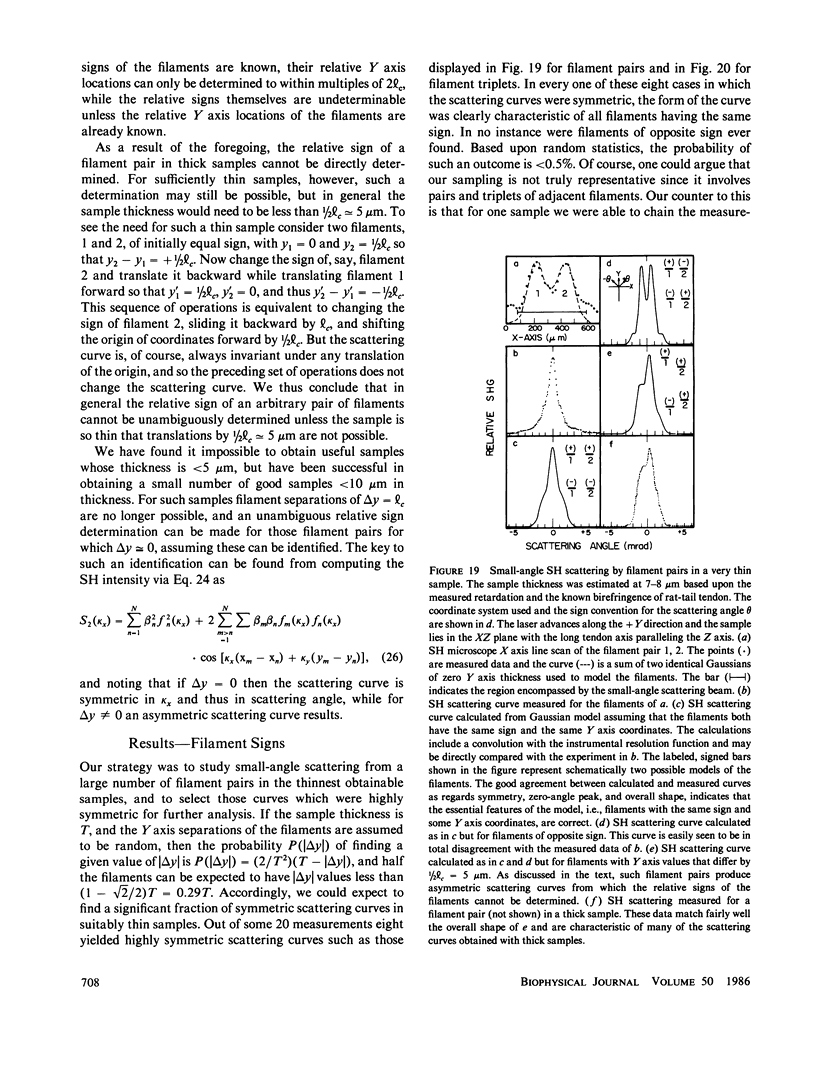
Connective tissue polarity has remained an intractable enigma for over two decades. We present new data on optical second harmonic generation in native, wet, rat-tail tendon. Scanning second-harmonic microscopy has revealed, for the first time, the existence of a discrete network of fine, polar, filamentous or columnar, structures, and, also, the presence of strongly polar surface, or near-surface patches. The thickness of these features was probed via crossed-beam optical frequency summation and the polar material is estimated to occupy a few percent of the tendon volume. The three-dimensional spatial distribution of filaments was studied with the aid of small-angle second-harmonic scattering, and the filaments were found to permeate the tendon cross-section in an apparently random fashion. These latter measurements also revealed that essentially all polar filaments had the same directionality. Concomitant studies of the polar collagen fibrils that comprise the bulk of tendon were in full accord with prior electron microscope results that had demonstrated that the directionality of these fibrils varies up/down in a purely random fashion, and thus cannot yield a net macroscopic polarity. Quantitative analysis of the second-harmonic data yields the conclusion that the observed polar structures cannot be simply local regions containing some accidental net excess of similarly oriented fibrils. The analytical expressions used in the analysis of the data obtained for this complex tissue were supported by extensive, realistic computer simulations. The discovery that the polarity of rat-tail tendon, and possibly other forms of connective tissue, resides in discrete structures, some of which are located near the tendon surface, should permit the ready isolation of polar-rich material for further study by a variety of techniques.
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. C., Eriksson C. Electrical properties of wet collagen. Nature. 1968 Apr 13;218(5137):166–168. doi: 10.1038/218166a0. [DOI] [PubMed] [Google Scholar]
- Athenstaedt H. Permanent longitudinal electric polarization and pyroelectric behaviour of collagenous structures and nervous tissue in man and other vertebrates. Nature. 1970 Nov 28;228(5274):830–834. doi: 10.1038/228830a0. [DOI] [PubMed] [Google Scholar]
- Athenstaedt H. Pyroelectric and piezoelectric properties of vertebrates. Ann N Y Acad Sci. 1974;238:68–94. doi: 10.1111/j.1749-6632.1974.tb26780.x. [DOI] [PubMed] [Google Scholar]
- Cusack S., Miller A. Determination of the elastic constants of collagen by Brillouin light scattering. J Mol Biol. 1979 Nov 25;135(1):39–51. doi: 10.1016/0022-2836(79)90339-5. [DOI] [PubMed] [Google Scholar]
- Diamant J., Keller A., Baer E., Litt M., Arridge R. G. Collagen; ultrastructure and its relation to mechanical properties as a function of ageing. Proc R Soc Lond B Biol Sci. 1972 Mar 14;180(1060):293–315. doi: 10.1098/rspb.1972.0019. [DOI] [PubMed] [Google Scholar]
- Jelinski L. W., Sullivan C. E., Torchia D. A. 2H NMR study of molecular motion in collagen fibrils. Nature. 1980 Apr 10;284(5756):531–534. doi: 10.1038/284531a0. [DOI] [PubMed] [Google Scholar]
- Jelinski L. W., Torchia D. A. 13C/1H high power double magnetic resonance investigation of collagen backbone motion in fibrils and in solution. J Mol Biol. 1979 Sep 5;133(1):45–65. doi: 10.1016/0022-2836(79)90250-x. [DOI] [PubMed] [Google Scholar]
- Jelinski L. W., Torchia D. A. Investigation of labeled amino acid side-chain motion in collagen using 13C nuclear magnetic resonance. J Mol Biol. 1980 Apr;138(2):255–272. doi: 10.1016/0022-2836(80)90286-7. [DOI] [PubMed] [Google Scholar]
- Parry D. A., Craig A. S. Quantitative electron microscope observations of the collagen fibrils in rat-tail tendon. Biopolymers. 1977 May;16(5):1015–1031. doi: 10.1002/bip.1977.360160506. [DOI] [PubMed] [Google Scholar]
- Roth S., Freund I. Optical second-harmonic scattering in rat-tail tendon. Biopolymers. 1981 Jun;20(6):1271–1290. doi: 10.1002/bip.1981.360200613. [DOI] [PubMed] [Google Scholar]
- Traub W., Piez K. A. The chemistry and structure of collagen. Adv Protein Chem. 1971;25:243–352. doi: 10.1016/s0065-3233(08)60281-8. [DOI] [PubMed] [Google Scholar]



