Abstract
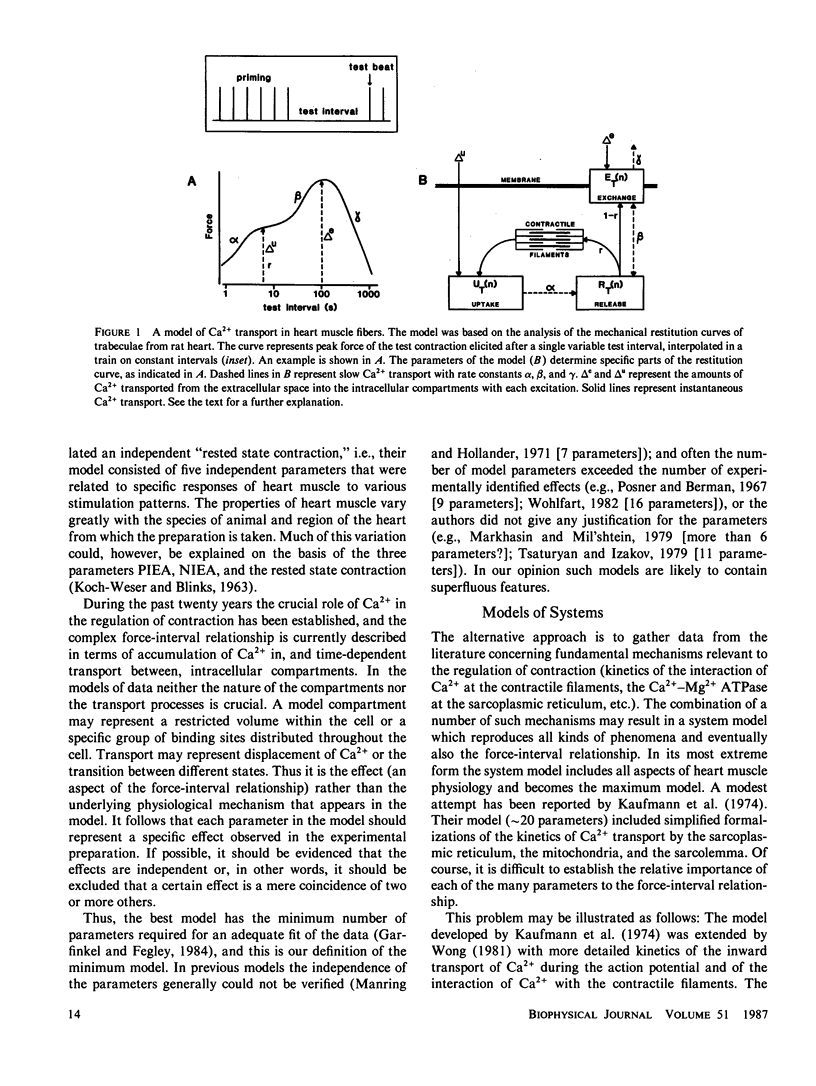
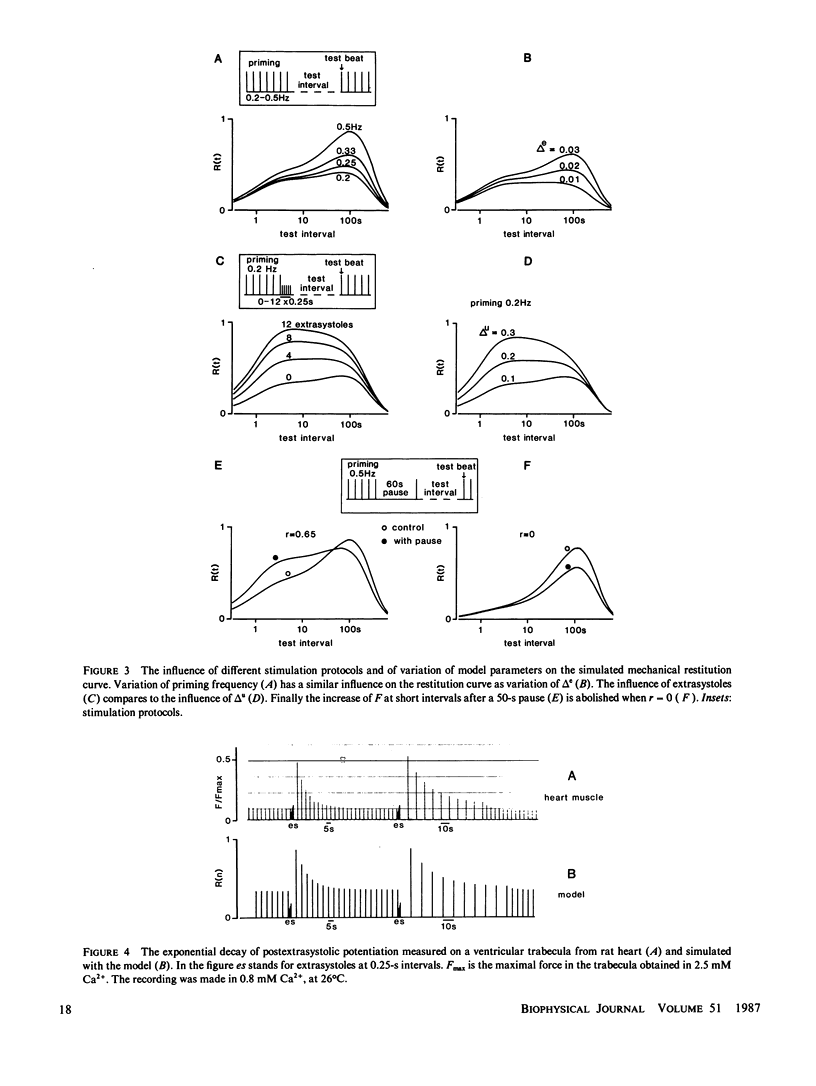
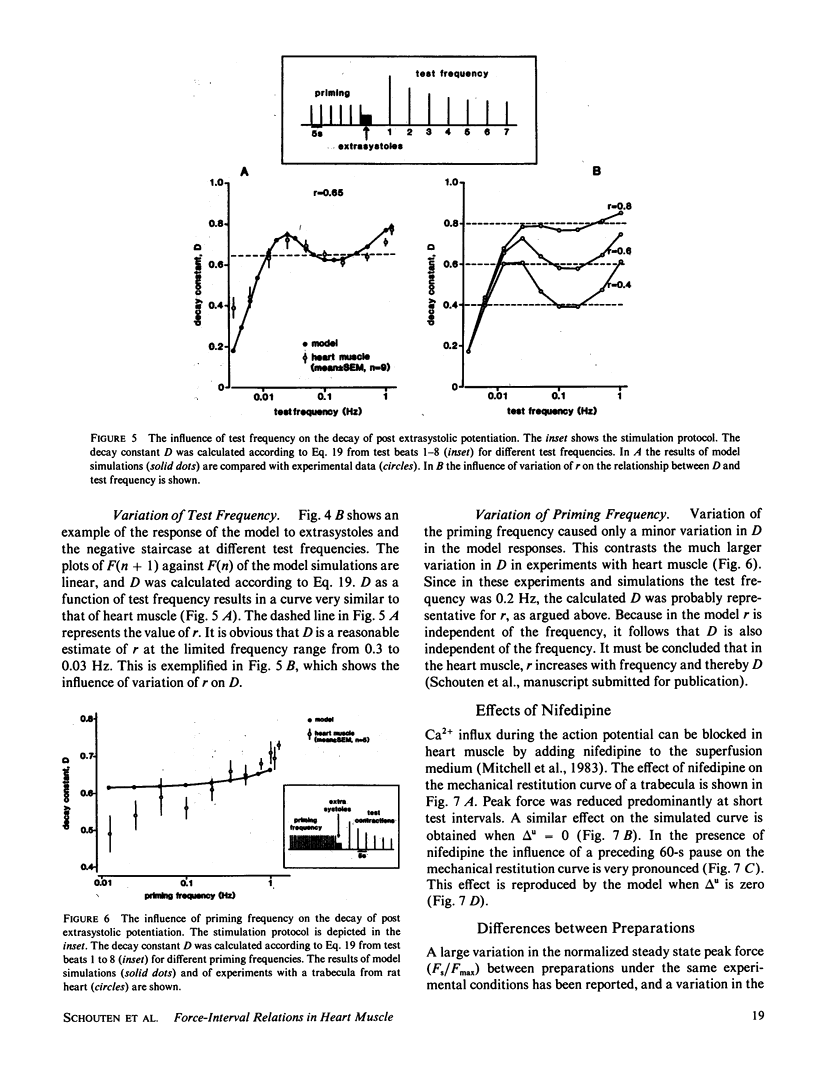
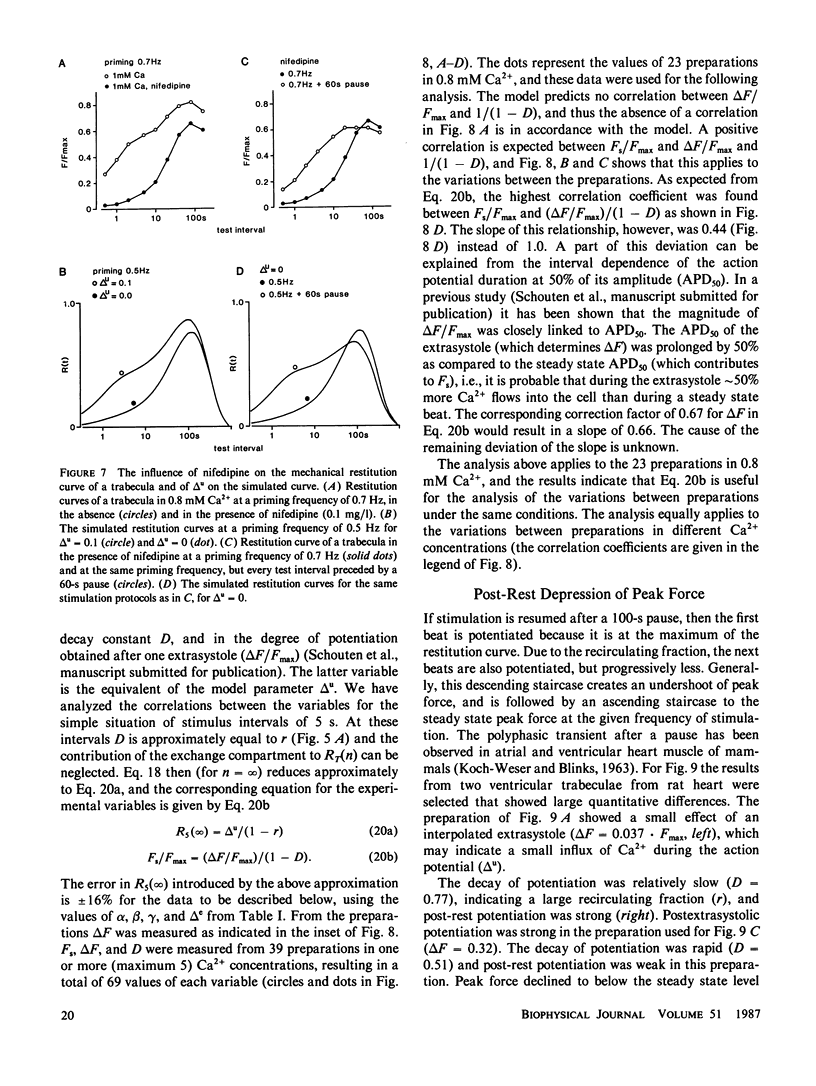
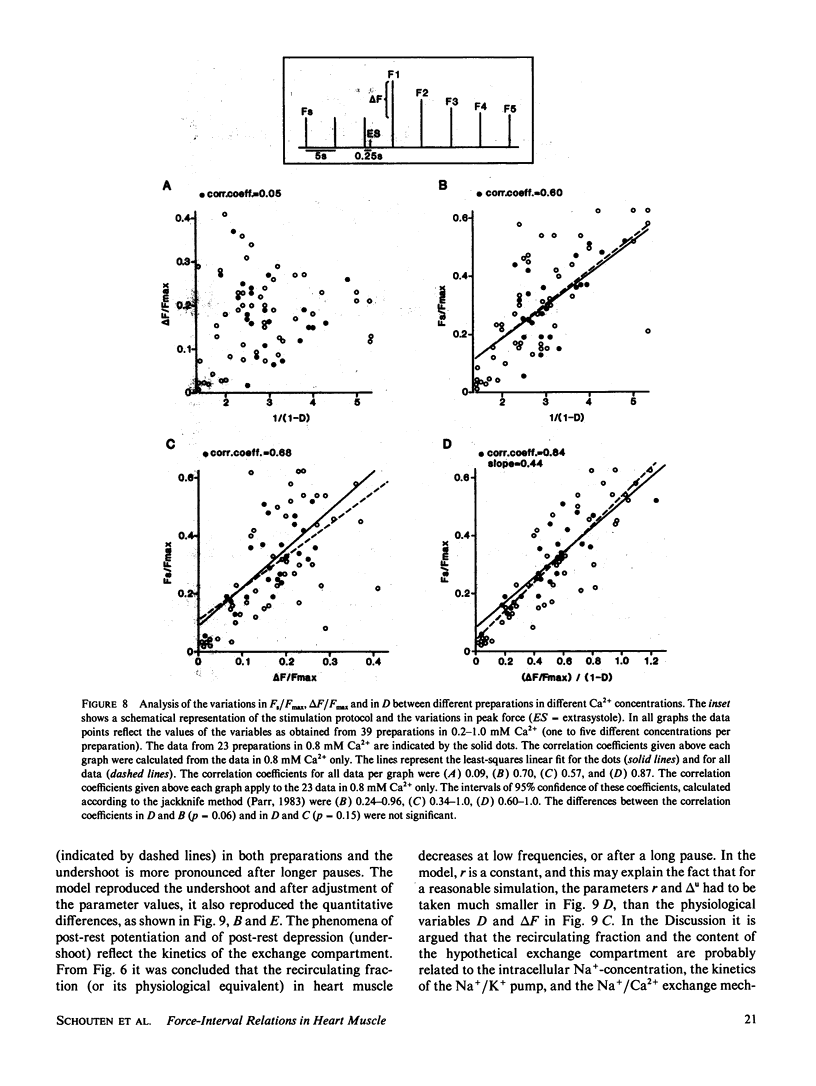
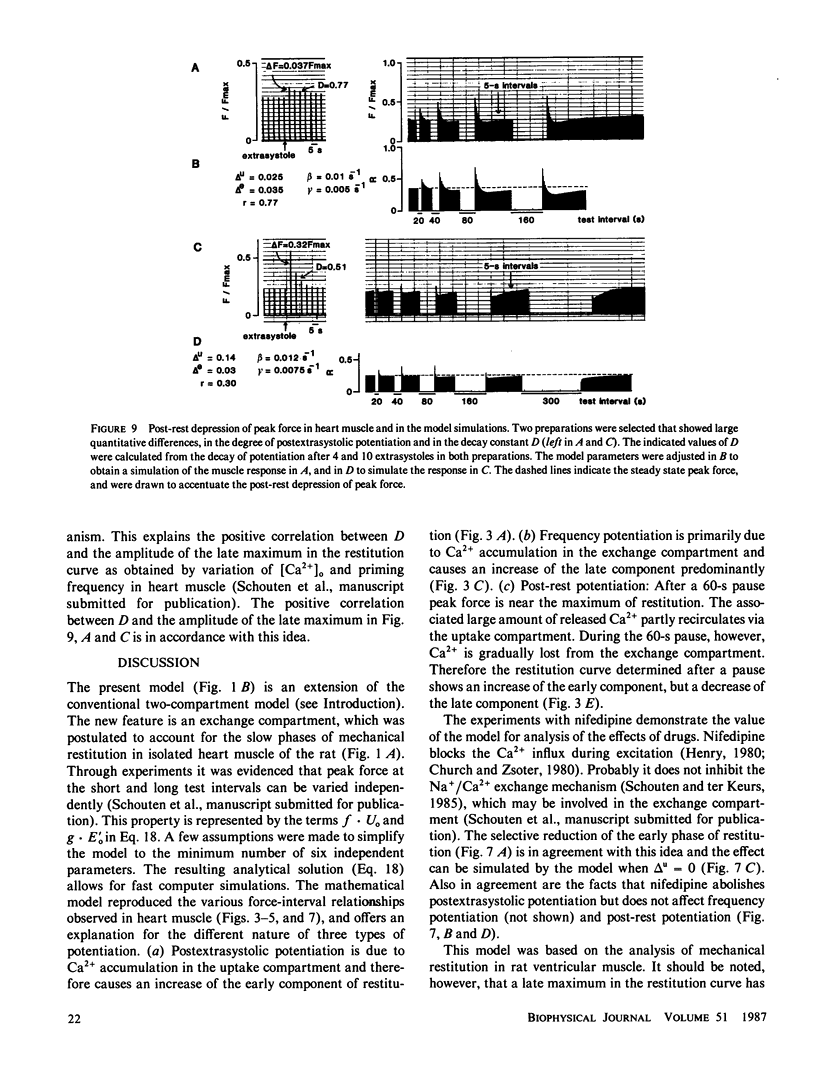
A mathematical model was derived that describes peak force of contraction as a function of stimulus interval and stimulus number in terms of Ca2+ transport between three hypothetical Ca2+ compartments. It includes the conventional uptake and release compartments and recirculation of a fraction r of the activator Ca2+. Peak force is assumed to be proportional to the amount of activator Ca2+ released from the release compartment into the sarcoplasm. A new extension is a slow exchange of Ca2+ with the extracellular space via an exchange compartment. Six independent parameters were necessary to reproduce the different effects of postextrasystolic potentiation, frequency potentiation, and post-rest potentiation in isolated heart muscle from the rat. The normalized steady state peak force (F/Fmax) under standard conditions varied by a factor of ten between preparations from rat heart. Analysis with the model indicated that most of this variation was caused by two variables: the Ca2+ influx per excitation and the recirculating fraction of activator Ca2+. The influence of the Ca2+ antagonist nifedipine of the force-interval relationship was reproduced by the model. It is concluded that the model may serve to analyze the variability of contractile force and the mode of actions of drugs in heart muscle.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler D., Wong A. Y., Mahler Y., Klassen G. A. Model of calcium movements in the mammalian myocardium: interval-strength relationship. J Theor Biol. 1985 Mar 21;113(2):379–394. doi: 10.1016/s0022-5193(85)80233-2. [DOI] [PubMed] [Google Scholar]
- Allen D. G., Jewell B. R., Wood E. H. Studies of the contractility of mammalian myocardium at low rates of stimulation. J Physiol. 1976 Jan;254(1):1–17. doi: 10.1113/jphysiol.1976.sp011217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni H., Jacob R., Kaufmann R. Mechanische Reaktionen des Frosch- und Säugetiermyokards bei Veränderung der Aktionspotential-Dauer durch konstante Gleichstromimpulse. Pflugers Arch. 1969;306(1):33–57. doi: 10.1007/BF00586610. [DOI] [PubMed] [Google Scholar]
- BLINKS J. R., KOCH-WESER J. Analysis of the effects of changes in rate and rhythm upon myocardial contractility. J Pharmacol Exp Ther. 1961 Dec;134:373–389. [PubMed] [Google Scholar]
- Bass O. The decay of the potentiated state in sheep and calf ventricular myocardial fibers. Influence of agents acting on transmembrane Ca2+ flux. Circ Res. 1976 Sep;39(3):396–399. doi: 10.1161/01.res.39.3.396. [DOI] [PubMed] [Google Scholar]
- Bassingthwaighte J. B., Fry C. H., McGuigan J. A. Relationship between internal calcium and outward current in mammalian ventricular muscle; a mechanism for the control of the action potential duration? J Physiol. 1976 Oct;262(1):15–37. doi: 10.1113/jphysiol.1976.sp011583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravený P., Sumbera J. Electromechanical correlations in the mammalian heart muscle. Pflugers Arch. 1970;319(1):36–48. doi: 10.1007/BF00586426. [DOI] [PubMed] [Google Scholar]
- Carafoli E. The homeostasis of calcium in heart cells. J Mol Cell Cardiol. 1985 Mar;17(3):203–212. doi: 10.1016/s0022-2828(85)80003-1. [DOI] [PubMed] [Google Scholar]
- Chapman R. A., Coray A., McGuigan J. A. Sodium/calcium exchange in mammalian ventricular muscle: a study with sodium-sensitive micro-electrodes. J Physiol. 1983 Oct;343:253–276. doi: 10.1113/jphysiol.1983.sp014891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church J., Zsotér T. T. Calcium antagonistic drugs. Mechanism of action. Can J Physiol Pharmacol. 1980 Mar;58(3):254–264. doi: 10.1139/y80-044. [DOI] [PubMed] [Google Scholar]
- Cohen C. J., Fozzard H. A., Sheu S. S. Increase in intracellular sodium ion activity during stimulation in mammalian cardiac muscle. Circ Res. 1982 May;50(5):651–662. doi: 10.1161/01.res.50.5.651. [DOI] [PubMed] [Google Scholar]
- Daut J. The role of intracellular sodium ions in the regulation of cardiac contractility. J Mol Cell Cardiol. 1982 Mar;14(3):189–192. doi: 10.1016/0022-2828(82)90119-5. [DOI] [PubMed] [Google Scholar]
- DiStefano J. J., 3rd, Landaw E. M. Multiexponential, multicompartmental, and noncompartmental modeling. I. Methodological limitations and physiological interpretations. Am J Physiol. 1984 May;246(5 Pt 2):R651–R664. doi: 10.1152/ajpregu.1984.246.5.R651. [DOI] [PubMed] [Google Scholar]
- Edman K. A., Jóhannsson M. The contractile state of rabbit papillary muscle in relation to stimulation frequency. J Physiol. 1976 Jan;254(3):565–581. doi: 10.1113/jphysiol.1976.sp011247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga G., Lab M. J., Noble M. I., Papadoyannis D. E., Pidgeon J., Seed A., Wohlfart B. The action-potential duration and contractile response of the intact heart related to the preceding interval and the preceding beat in the dog and cat. J Physiol. 1981 May;314:481–500. doi: 10.1113/jphysiol.1981.sp013720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983 Jul;245(1):C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Fozzard H. A. Heart: excitation-contraction coupling. Annu Rev Physiol. 1977;39:201–220. doi: 10.1146/annurev.ph.39.030177.001221. [DOI] [PubMed] [Google Scholar]
- Gadsby D. C., Cranefield P. F. Electrogenic sodium extrusion in cardiac Purkinje fibers. J Gen Physiol. 1979 Jun;73(6):819–837. doi: 10.1085/jgp.73.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel D., Fegley K. A. Fitting physiological models to data. Am J Physiol. 1984 May;246(5 Pt 2):R641–R650. doi: 10.1152/ajpregu.1984.246.5.R641. [DOI] [PubMed] [Google Scholar]
- HOFFMAN B. F., BINDLER E., SUCKLING E. E. Postextrasystolic potentiation of contraction in cardiac muscle. Am J Physiol. 1956 Apr;185(1):95–102. doi: 10.1152/ajplegacy.1956.185.1.95. [DOI] [PubMed] [Google Scholar]
- Henry P. D. Comparative pharmacology of calcium antagonists: nifedipine, verapamil and diltiazem. Am J Cardiol. 1980 Dec 1;46(6):1047–1058. doi: 10.1016/0002-9149(80)90366-5. [DOI] [PubMed] [Google Scholar]
- Hilgemann D. W., Delay M. J., Langer G. A. Activation-dependent cumulative depletions of extracellular free calcium in guinea pig atrium measured with antipyrylazo III and tetramethylmurexide. Circ Res. 1983 Dec;53(6):779–793. doi: 10.1161/01.res.53.6.779. [DOI] [PubMed] [Google Scholar]
- Johnson E. A., Kootsey J. M. A minimum mechanism for Na+-Ca++ exchange: net and unidirectional Ca++ fluxes as functions of ion composition and membrane potential. J Membr Biol. 1985;86(2):167–187. doi: 10.1007/BF01870783. [DOI] [PubMed] [Google Scholar]
- Josephson I. R., Sanchez-Chapula J., Brown A. M. A comparison of calcium currents in rat and guinea pig single ventricular cells. Circ Res. 1984 Feb;54(2):144–156. doi: 10.1161/01.res.54.2.144. [DOI] [PubMed] [Google Scholar]
- KOCH-WESER J., BLINKS J. R. THE INFLUENCE OF THE INTERVAL BETWEEN BEATS ON MYOCARDIAL CONTRACTILITY. Pharmacol Rev. 1963 Sep;15:601–652. [PubMed] [Google Scholar]
- Katz A. M. "Tonic" and "phasic" mechanisms in the regulation of myocardial contractility. Basic Res Cardiol. 1976 Sep-Oct;71(5):447–455. doi: 10.1007/BF01909761. [DOI] [PubMed] [Google Scholar]
- Kaufmann R., Bayer R., Fürniss T., Krause H., Tritthart H. Calcium-movement controlling cardiac contractility II. Analog computation of cardiac excitation-contraction coupling on the basis of calcium kinetics in a multi-compartment model. J Mol Cell Cardiol. 1974 Dec;6(6):543–559. doi: 10.1016/0022-2828(74)90035-2. [DOI] [PubMed] [Google Scholar]
- Landau M., Valentini F. A. Construction, mathematical study and numerical simulation of a calcium turnover model during skeletal muscle contraction. Int J Biomed Comput. 1982 Jan;13(1):49–68. doi: 10.1016/0020-7101(82)90050-2. [DOI] [PubMed] [Google Scholar]
- Lee C. O., Dagostino M. Effect of strophanthidin on intracellular Na ion activity and twitch tension of constantly driven canine cardiac Purkinje fibers. Biophys J. 1982 Dec;40(3):185–198. doi: 10.1016/S0006-3495(82)84474-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manring A., Hollander P. B. The interval-strength relationship in mammalian atrium: a calcium exchange model. I. Theory. Biophys J. 1971 Jun;11(6):483–501. doi: 10.1016/S0006-3495(71)86230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. Characteristics of the second inward current in cells isolated from rat ventricular muscle. Proc R Soc Lond B Biol Sci. 1983 Oct 22;219(1217):447–469. doi: 10.1098/rspb.1983.0084. [DOI] [PubMed] [Google Scholar]
- Mullins L. J. A mechanism for Na/Ca transport. J Gen Physiol. 1977 Dec;70(6):681–695. doi: 10.1085/jgp.70.6.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins L. J. The generation of electric currents in cardiac fibers by Na/Ca exchange. Am J Physiol. 1979 Mar;236(3):C103–C110. doi: 10.1152/ajpcell.1979.236.3.C103. [DOI] [PubMed] [Google Scholar]
- Posner C. J., Berman D. A. A mathematical analysis of the interval-strength relationship in the rat ventricle strip and its modification by fluoroacetate. J Pharmacol Exp Ther. 1967 Apr;156(1):166–177. [PubMed] [Google Scholar]
- Ragnarsdóttir K., Wohlfart B., Jóhannsson M. Mechanical restitution of the rat papillary muscle. Acta Physiol Scand. 1982 Jun;115(2):183–191. doi: 10.1111/j.1748-1716.1982.tb07064.x. [DOI] [PubMed] [Google Scholar]
- Reuter H. Exchange of calcium ions in the mammalian myocardium. Mechanisms and physiological significance. Circ Res. 1974 May;34(5):599–605. doi: 10.1161/01.res.34.5.599. [DOI] [PubMed] [Google Scholar]
- SCRIABINE A. [On the change of the contraction amplitude of electrically stimulated rat papillary muscle after a stimulation pause or a period of high frequency stimulation]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1959;269:311–318. [PubMed] [Google Scholar]
- Schouten V. J. The relationship between action potential duration and force of contraction in rat myocardium. Eur Heart J. 1984 Dec;5(12):984–992. doi: 10.1093/oxfordjournals.eurheartj.a061618. [DOI] [PubMed] [Google Scholar]
- Schouten V. J., ter Keurs H. E. The slow repolarization phase of the action potential in rat heart. J Physiol. 1985 Mar;360:13–25. doi: 10.1113/jphysiol.1985.sp015601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu S. S., Fozzard H. A. Transmembrane Na+ and Ca2+ electrochemical gradients in cardiac muscle and their relationship to force development. J Gen Physiol. 1982 Sep;80(3):325–351. doi: 10.1085/jgp.80.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallinga-de Jonge W., Boom H. B., Heijink R. J., van der Vliet G. H. Calcium model for mammalian skeletal muscle. Med Biol Eng Comput. 1981 Nov;19(6):734–748. doi: 10.1007/BF02441335. [DOI] [PubMed] [Google Scholar]
- Wohlfart B., Elzinga G. Electrical and mechanical responses of the intact rabbit heart in relation to the excitation interval. A comparison with the isolated papillary muscle preparation. Acta Physiol Scand. 1982 Jul;115(3):331–340. doi: 10.1111/j.1748-1716.1982.tb07086.x. [DOI] [PubMed] [Google Scholar]
- Wohlfart B., Noble M. I. The cardiac excitation-contraction cycle. Pharmacol Ther. 1982;16(1):1–43. doi: 10.1016/0163-7258(82)90030-4. [DOI] [PubMed] [Google Scholar]
- Wohlfart B. Relationships between peak force, action potential duration and stimulus interval in rabbit myocardium. Acta Physiol Scand. 1979 Aug;106(4):395–409. doi: 10.1111/j.1748-1716.1979.tb06419.x. [DOI] [PubMed] [Google Scholar]
- Wong A. Y. A model of excitation-contraction coupling of mammalian cardiac muscle. J Theor Biol. 1981 May 7;90(1):37–61. doi: 10.1016/0022-5193(81)90121-1. [DOI] [PubMed] [Google Scholar]
- Yellin E. L., Kennish A., Yoran C., Laniado S., Buckley N. M., Frater R. W. The influence of left ventricular filling on postextrasystolic potentiation in the dog heart. Circ Res. 1979 May;44(5):712–722. doi: 10.1161/01.res.44.5.712. [DOI] [PubMed] [Google Scholar]


