Abstract
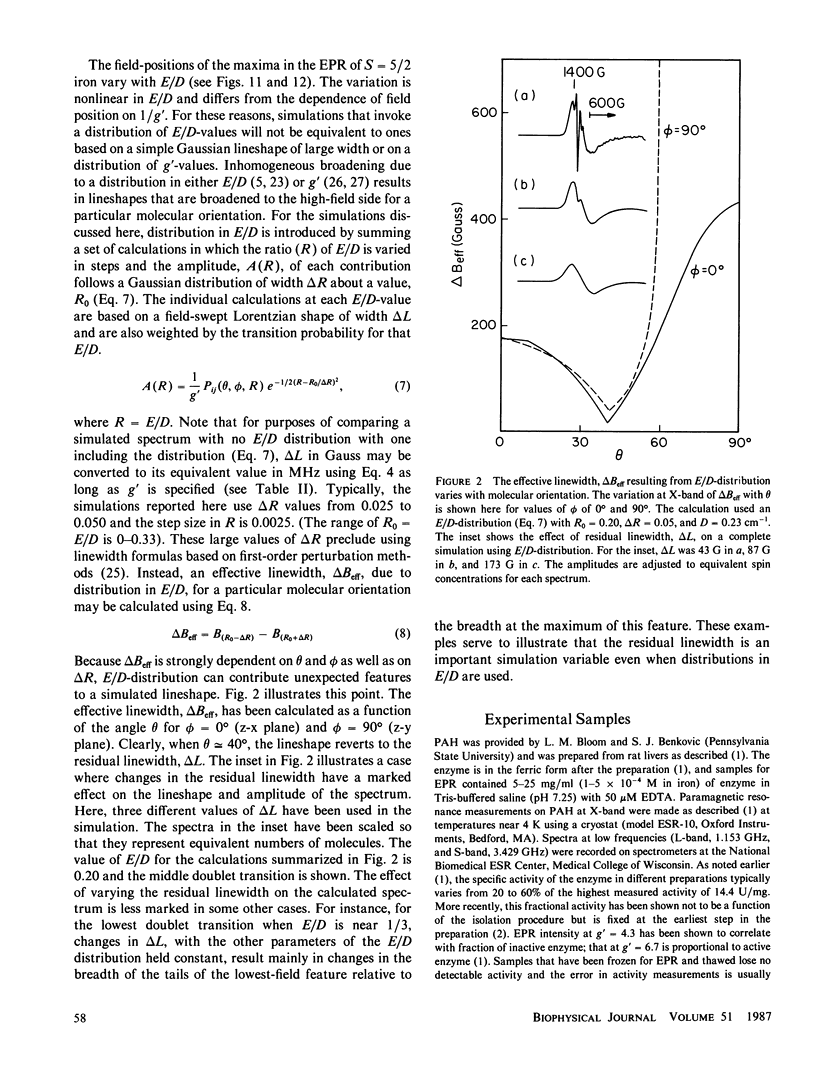
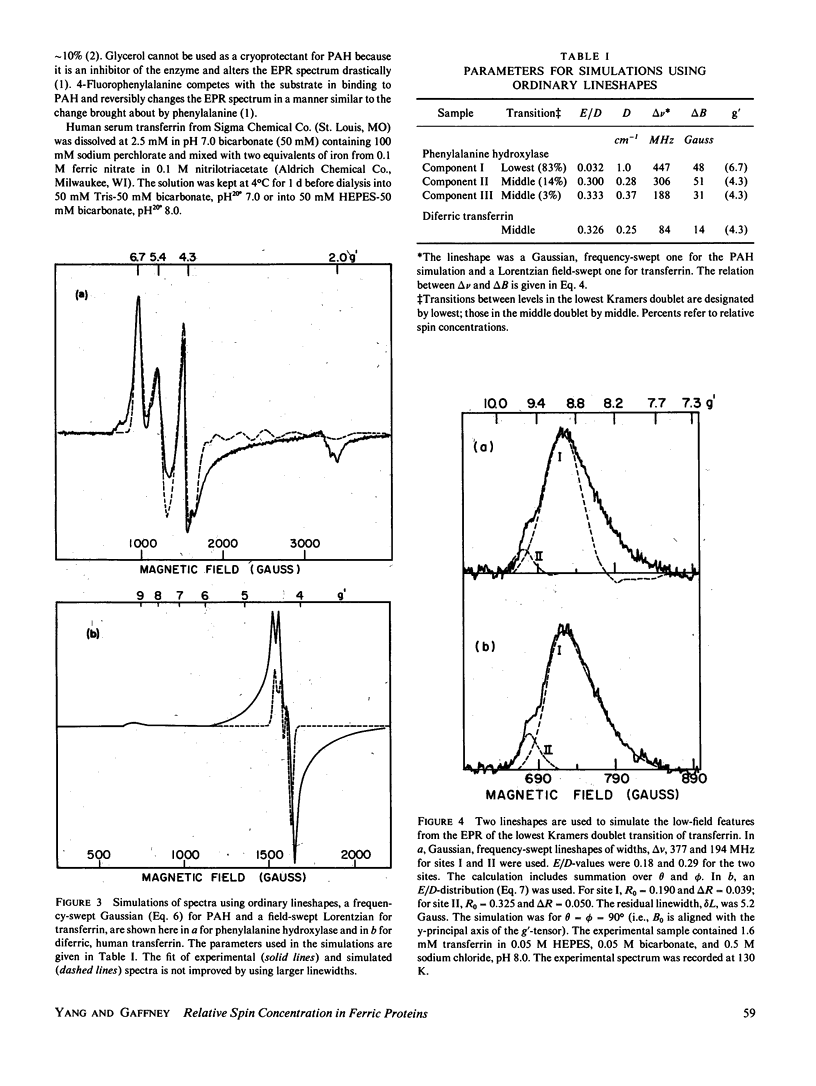
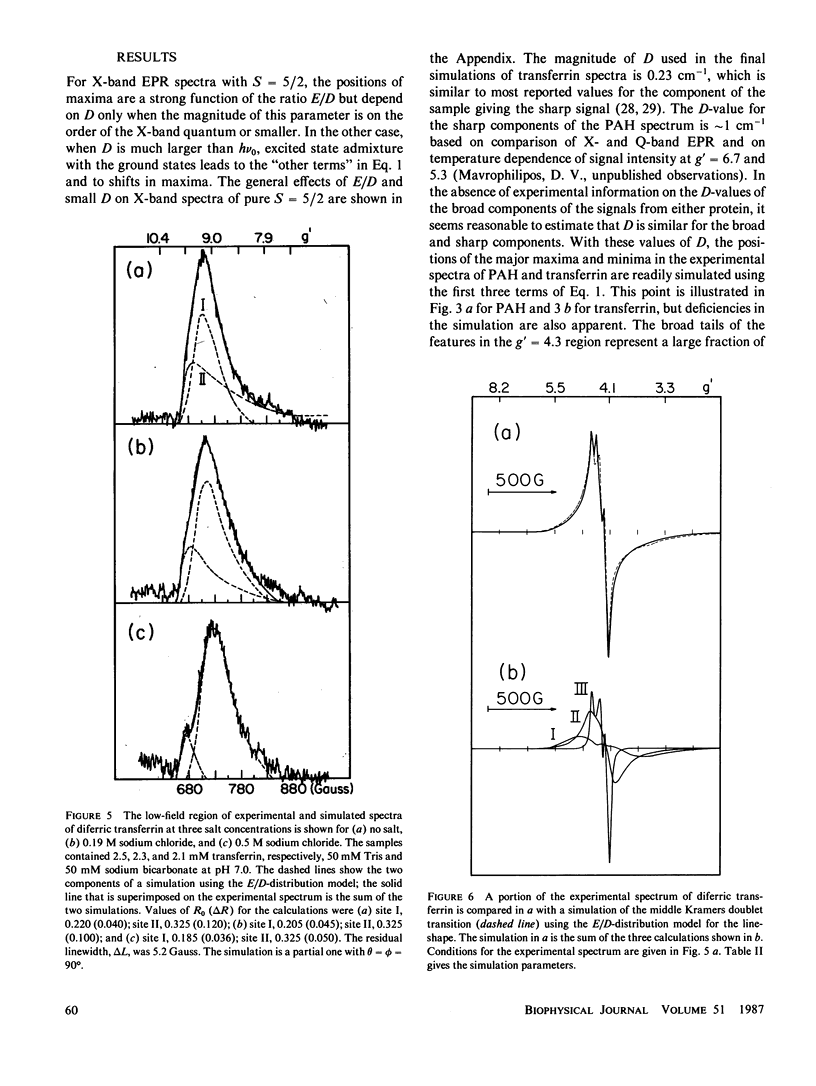
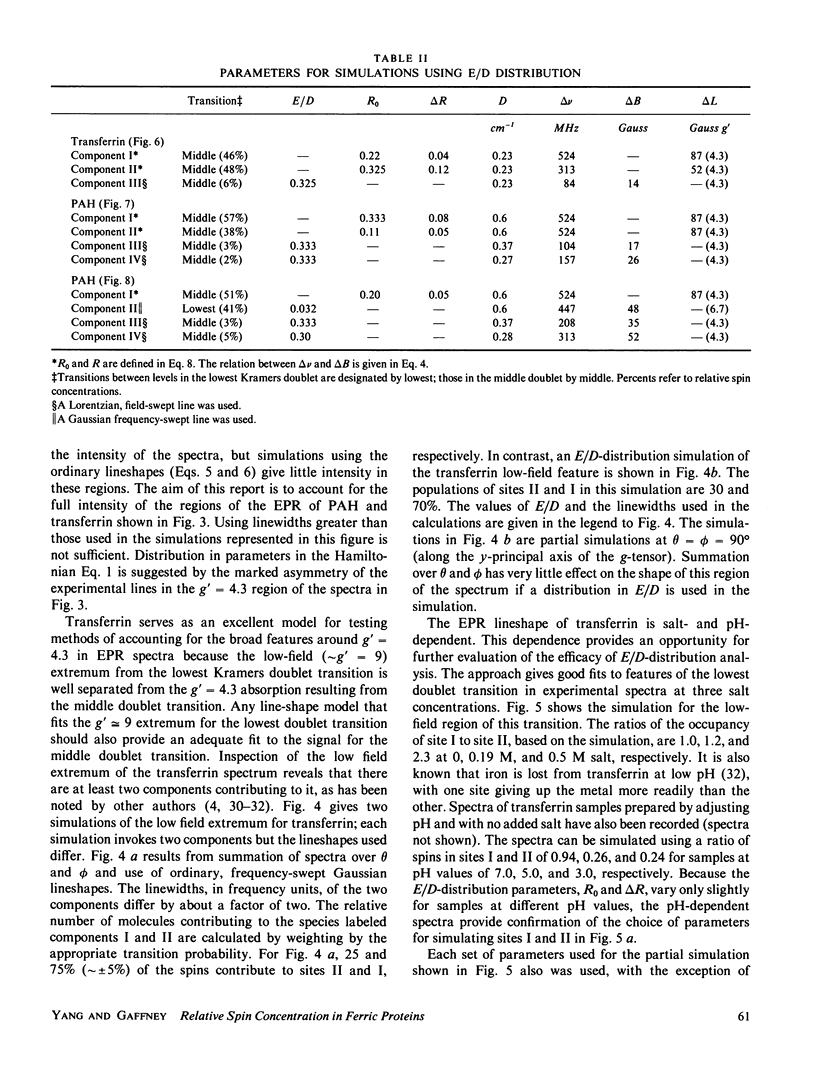
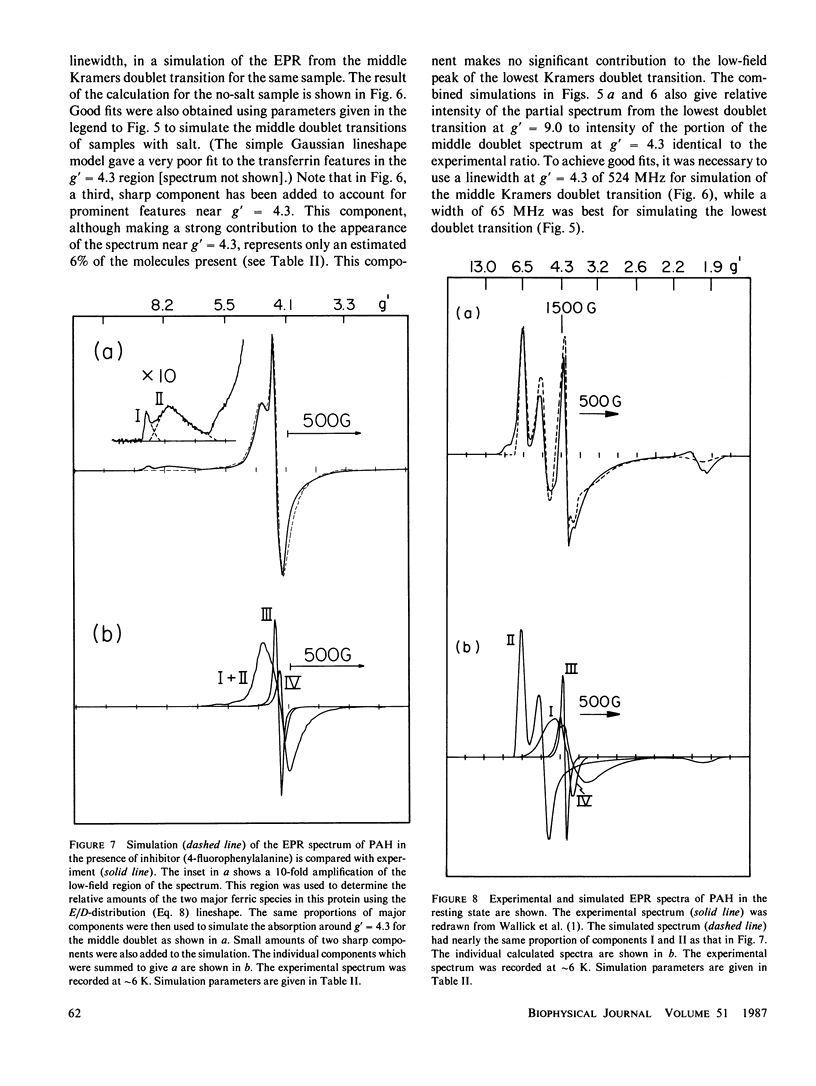
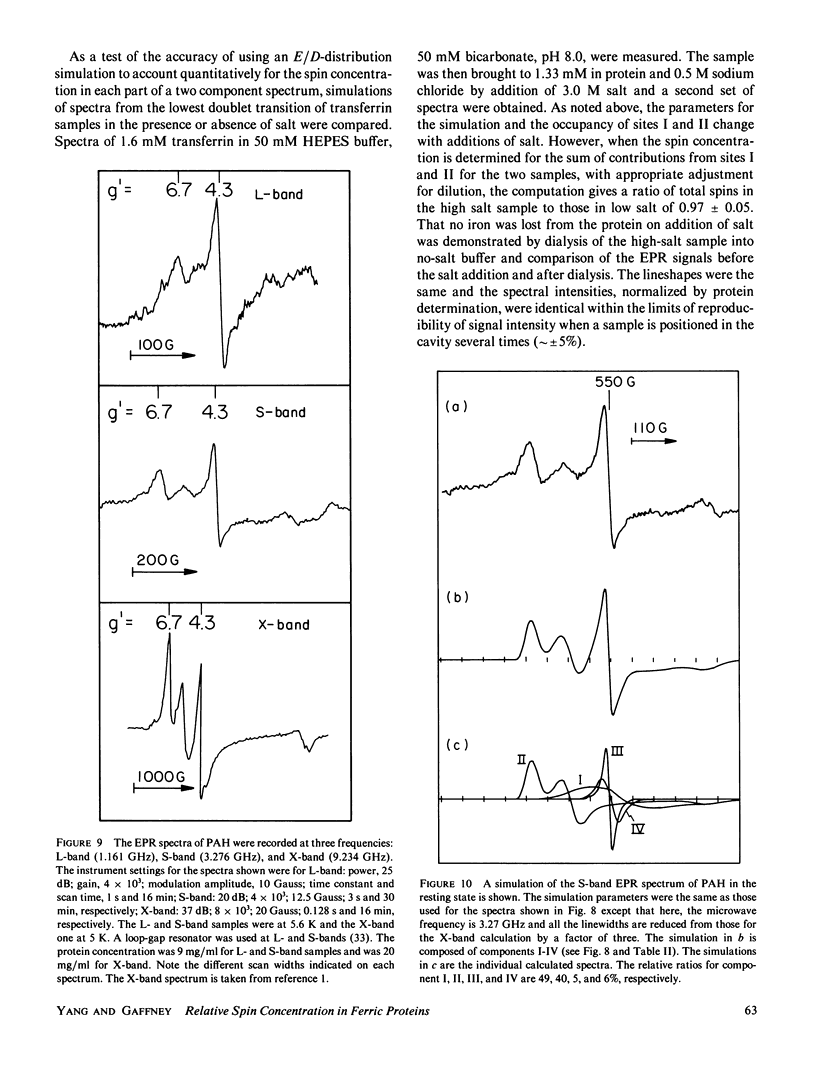
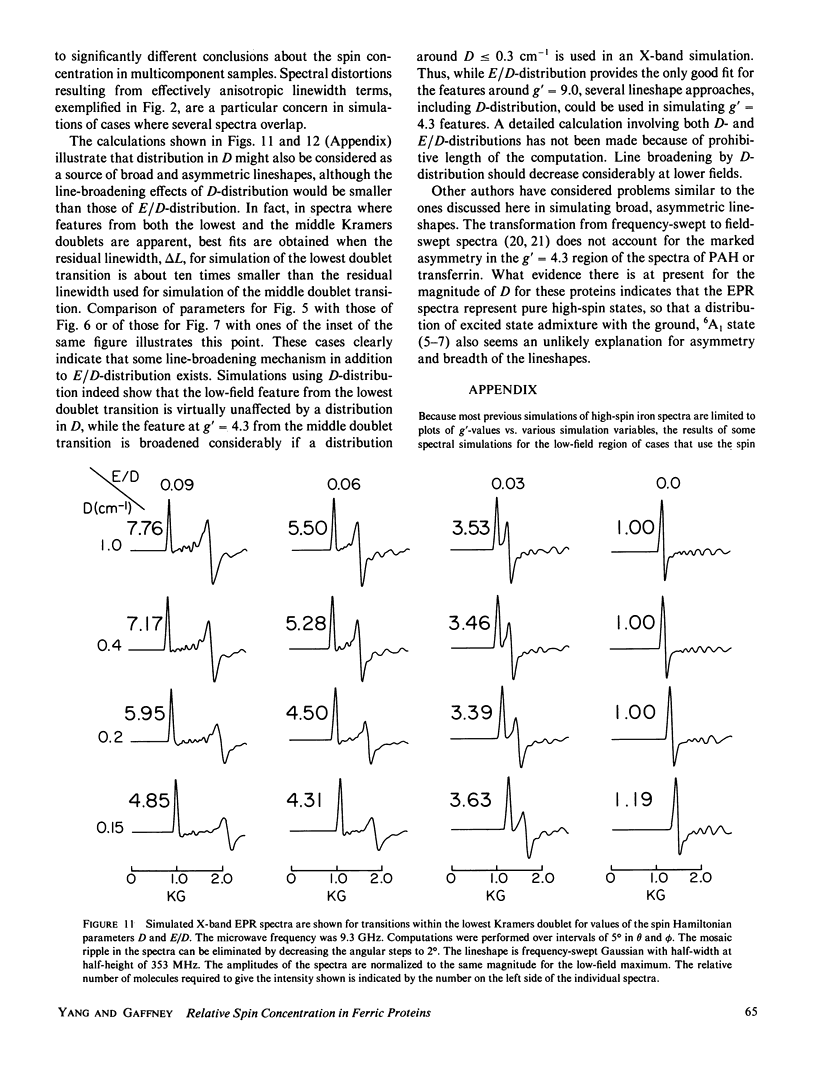
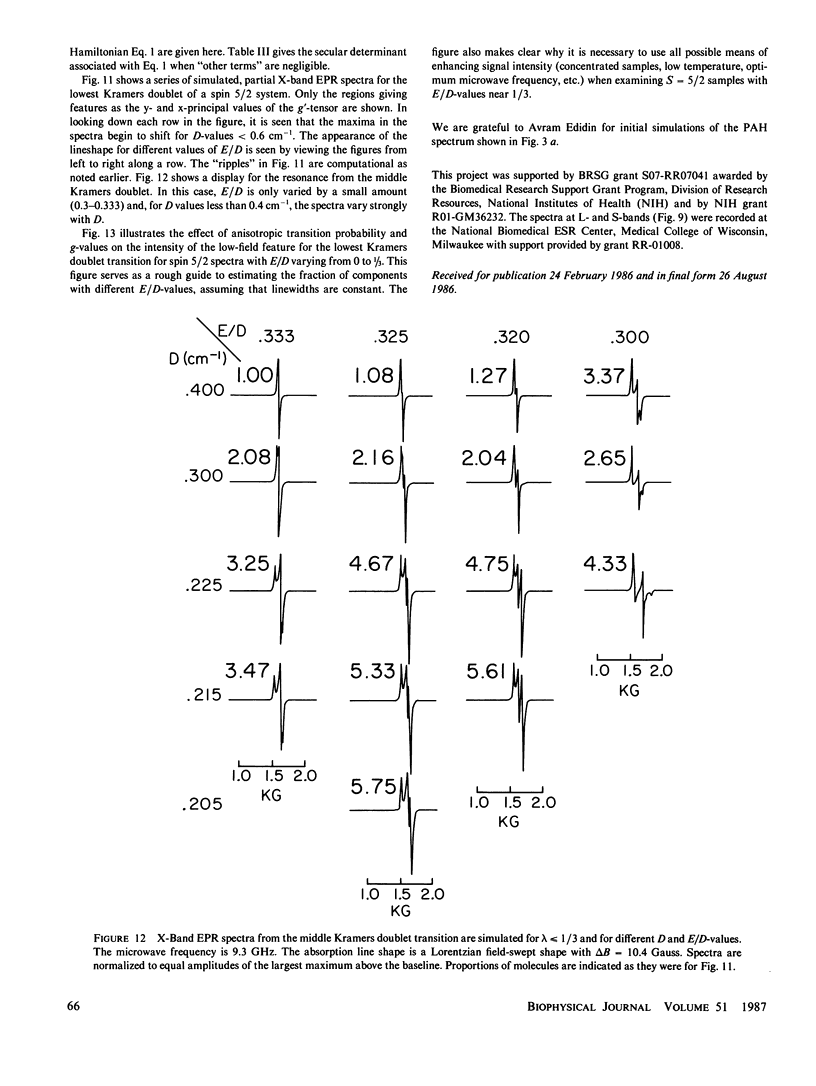
Lineshape simulations are presented for the multiple, overlapping X-band electron paramagnetic resonance (EPR) spectra in two non-heme, high-spin iron proteins: phenylalanine hydroxylase (PAH) and diferric transferrin. The aim of the calculations is to determine the fraction of iron contributing to each of the sites visible by EPR. The simulations are limited to the experimentally accessible transitions occurring at g-values greater than 1.7. In both PAH and transferrin, at least one of the iron sites is characterized by the ratio of zero-field splitting parameters, E/D, near 1/3 and a broad, asymmetric lineshape. A distribution in E/D-values is used in the simulations to account for this breadth and asymmetry. To test the E/D-distribution model, experimental X-band spectra of diferric transferrin at several salt concentrations are fit by simulation. In this test, first the low-field features arising from transitions between the lowest Kramers doublet levels are simulated using E/D-distributions for two sites. Second, parameters that provide a good fit for the lowest doublet transitions are shown also to fit the resonance near an effective g-value of 4.3 from the middle Kramers doublet transition. When applied to spectra of PAH in the resting state, the E/D-distribution approach accounts for the intensity of one of the two major species of iron. The other species is characterized by E/D = 0.032, and the spectrum of this portion of the resting enzyme may be simulated using a frequency-swept Gaussian lineshape. Spectra for the enzyme in an inhibitor-saturated state are also simulated. The simulations are consistent with previous biochemical studies that indicate that only the E/D = 0.032 form of iron participates in catalysis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aasa R. Re-interpretation of the electron paramagnetic resonance spectra of transferrins. Biochem Biophys Res Commun. 1972 Nov 1;49(3):806–812. doi: 10.1016/0006-291x(72)90482-2. [DOI] [PubMed] [Google Scholar]
- Aisen P., Leibman A., Zweier J. Stoichiometric and site characteristics of the binding of iron to human transferrin. J Biol Chem. 1978 Mar 25;253(6):1930–1937. [PubMed] [Google Scholar]
- Bloom L. M., Benkovic S. J., Gaffney B. J. Characterization of phenylalanine hydroxylase. Biochemistry. 1986 Jul 29;25(15):4204–4210. doi: 10.1021/bi00363a006. [DOI] [PubMed] [Google Scholar]
- Brill AS, Fiamingo FG, Hampton DA, Levin PD, Thorkildsen R. Density of low-energy vibrational states in a protein solution. Phys Rev Lett. 1985 Apr 22;54(16):1864–1867. doi: 10.1103/PhysRevLett.54.1864. [DOI] [PubMed] [Google Scholar]
- Maltempo M. M. The spin 3/2 state and quantum spin mixtures in haem proteins. Q Rev Biophys. 1976 May;9(2):181–215. doi: 10.1017/s0033583500002407. [DOI] [PubMed] [Google Scholar]
- Pinkowitz R. A., Aisen P. Zero-field splittings of iron complexes of transferrins. J Biol Chem. 1972 Dec 10;247(23):7830–7834. [PubMed] [Google Scholar]
- Price E. M., Gibson J. F. Electron paramagnetic resonance evidence for a distinction between the two iron-binding sites in transferrin and in conalbumin. J Biol Chem. 1972 Dec 25;247(24):8031–8035. [PubMed] [Google Scholar]
- Princiotto J. V., Zapolski E. J. Difference between the two iron-binding sites of transferrin. Nature. 1975 May 1;255(5503):87–88. doi: 10.1038/255087a0. [DOI] [PubMed] [Google Scholar]
- Wallick D. E., Bloom L. M., Gaffney B. J., Benkovic S. J. Reductive activation of phenylalanine hydroxylase and its effect on the redox state of the non-heme iron. Biochemistry. 1984 Mar 13;23(6):1295–1302. doi: 10.1021/bi00301a043. [DOI] [PubMed] [Google Scholar]



