Abstract
The guinea pig has been utilized as a model for studying infectious diseases because its reactions closely resemble those of humans biologically and immunologically. However, the cytokine responses in this animal remain to be studied. Initially, we established a quantitative assay using a real-time reverse transcription-PCR (RT-PCR) to measure guinea pig gamma interferon (IFN-γ), interleukin-12 (IL-12), IL-10, and transforming growth factor β (TGF-β) mRNA. By preparing primer-fluorogenic probe sets for these cytokines and standard RNA templates corresponding to the target sequence of each cytokine, we obtained linear standard curves essential for quantitative determination. In guinea pigs immunized by intradermal (i.d.) vaccination with the Tokyo strain of Mycobacterium bovis BCG (0.1 mg) or else hyperimmunized with the same vaccine (10 mg) given intravenously (i.v.), peripheral blood mononuclear cells (PBMCs) at 4 weeks showed an increase in IFN-γ mRNA expression in the latter but not the former animals. However, at week 10, IFN-γ mRNA expression was markedly elevated in PBMCs, spleen cells, and cells in bronchoalveolar lavage fluid in both the i.d.- and the i.v.-immunized animals, the level of expression being 10 times higher in the latter. In contrast, the expression levels of IL-12 mRNA in PBMCs, spleen cells, and BAL cells were not enhanced in either group at 10 weeks postimmunization. The expression of IL-10 and TGF-β increased slightly only in PBMCs. Regardless of differences in the levels of cytokine responses, the magnitudes of the purified protein derivative of tuberculin-specific delayed-type hypersensitivity (DTH) skin reactions for the two groups did not differ significantly at 8 weeks postvaccination. In this study, we quantitatively measured IL-10, IL-12, TGF-β, and IFN-γ mRNA in BCG-immunized guinea pigs and showed that the level of IFN-γ mRNA expression does not necessarily reflect the magnitude of the DTH response, suggesting that there may be an intricate relationship between protective immunity, the level of IFN-γ, and the DTH response. Thus, our quantitative assay would be of use for the development of vaccines using guinea pig models.
Mycobacterium tuberculosis is a cell-associated pathogen, found mainly in macrophages. To eliminate this pathogen from a host, it is considered essential to activate the macrophages. The macrophage is an antigen-presenting cell that facilitates the activation of CD4+ Th1 cells. These activated Th1 cells then induce the expression of Th1-type cytokines, especially gamma interferon (IFN-γ), in cooperation with interleukin-12 (IL-12), also produced by antigen-stimulated macrophages; the IFN-γ subsequently activates the macrophages (33). Thus, an important host response to M. tuberculosis is the production of Th1-type cytokines, particularly IFN-γ. Indeed, it has been reported that IFN-γ is crucial to the control of this pathogen (11, 16, 38).
The guinea pig has been utilized to study M. tuberculosis infections (8, 36) and assess the efficacy of vaccines against tuberculosis (25) because it is exquisitely susceptible to infection with human tubercle bacilli (1) and closely resembles humans biologically and immunologically (13, 42). Indeed, guinea pigs have been employed in the testing of many of the antibiotics currently used to treat tuberculosis (9, 37). Furthermore, the development and preclinical testing of the Mycobacterium bovis BCG vaccine, one of the most widely used vaccines in the world, were also performed by using this animal. However, one disadvantage of the guinea pig model is the shortage of readily available reagents for immunological experiments. Therefore, in spite of the good biological properties described above, the analysis of cytokines in this animal remains undeveloped. Once this obstacle is overcome, the guinea pig should be much more available for the study of infectious diseases. Scarozza. et al. determined the sequences of several cytokines other than IFN-γ in guinea pigs and examined the expression of the associated mRNA by the traditional agarose gel reverse transcription-PCR (RT-PCR) method using nonimmunized and noninfected guinea pigs (34).
Recently, a real-time quantitative RT-PCR method based on the 5′-3′ nuclease activity of DNA polymerases (24, 32) and Förster-type energy transfer (17) has emerged. When a fluorescence-labeled probe that specifically hybridizes the target sequence between two primers is cleaved off by the DNA polymerase during the primer extension phase, fluorescence is emitted and the subsequent increase in signal is monitored automatically. To make quantitative measurements with this system, it is necessary to create a quantified standard RNA template corresponding to the target sequence for the cytokine.
In the present study, using a fluorogenic real-time RT-PCR system, we attempted to establish a method for the quantitative determination of Th1-type (IFN-γ and IL-12) and Th2-type (IL-10 and transforming growth factor β [TGF-β]) cytokine mRNA expression in the guinea pig. Furthermore, we investigated the pattern of cytokine expression in guinea pigs immunized with 0.1 mg of the vaccine BCG-Tokyo intradermally (i.d.) or hyperimmunized with 10 mg of this vaccine intravenously (i.v.).
MATERIALS AND METHODS
Experimental animals.
Pathogen-free outbred Hartley female guinea pigs (Shizuoka Laboratory Center, Shizuoka, Japan), weighing 200 to 220 g, were used in this study.
Immunization.
One group (n = 5) of guinea pigs was immunized once with 0.1 mg of BCG strain Tokyo i.d., and another group (n = 5) was immunized with 10 mg of the same vaccine i.v. A nonimmunized group (n = 5) was used as a control.
Preparation of mononuclear cells and RNA extraction.
Mononuclear cells were isolated from the peripheral blood, spleens, and lungs of the immunized and nonimmunized guinea pigs. Peripheral blood mononuclear cells (PBMCs) were prepared with Lymphosepar (ILB Co., Ltd., Gunma, Japan) 4 and 10 weeks after vaccination with BCG-Tokyo. At week 10, all guinea pigs were sacrificed with ketamine hydrochloride (Sankyo Co., Ltd., Tokyo, Japan), and the spleens and lungs were removed for the isolation of lymphocytes; spleen cells were prepared by gentle homogenization with a 70-μm-pore-size nylon cell strainer (Becton Dickinson and Company, Franklin Lakes, N.J.); cells in bronchoalveolar lavage fluid (BAL cells) were isolated by washing out the inside of the lung with phosphate-buffered saline (PBS). Then, the preparations were treated with ACK lysing buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM Na2-EDTA) for 5 min at room temperature to destroy red blood cells, and the obtained cells were washed three times in PBS.
The cells described above were adjusted to 0.5 × 107 to 1.0 × 107/ml in RPMI 1640 medium supplemented with 10% fetal calf serum and 50 μg of streptomycin, 50 U of penicillin, and 10 μg of gentamicin/ml and then cultured with or without 100 μg of purified protein derivative (PPD) of tuberculin antigen/ml at 37°C for 4 days. Following the culture, total cellular RNA was extracted according to the instructions with the RNeasy minikit (Qiagen, Valencia, Calif.) and stored at −80°C.
Oligonucleotide primers and probes.
The information about guinea pig IL-10, IL-12, and TGF-β sequences was obtained from the GenBank database (accession no. AF097510, AF097507, and AF097509). The guinea pig IFN-γ sequence was kindly supplied by T. Yoshimura (Frederick Cancer Research and Development Center, National Cancer Institute, National Institutes of Health). The primer-probe sets for these cytokines for real-time RT-PCR were designed by using Primer Express software (PE Applied Biosystems, Foster City, Calif.) according to the manufacturer's guidelines and synthesized by the oligonucleotide factory of Hokkaido System Science Co., Ltd. (Hokkaido, Japan). The sequences for primers and fluorescence-labeled probes are listed in Table 1.
TABLE 1.
Quantitative RT-PCR primers and probes
| Cytokine | Sequences (5′→3′)a |
|---|---|
| IFN-γ | (+)-CATGAACACCATCAAGGAACAAAT, (−)-TTTGAATCAGGTTTTTGAAAGCC, FAMb-TTCAAAGACAACAGCAGCAACAAGGTGC-TAMRAc |
| IL-10 | (+)-CAGCCTTGCAGAAAAGAGAGC, (−)-CCAGTAAGGCCAGGCAACAT, FAM-CATCATGCCTGGCTCAGCACTGC-TAMRA |
| IL-12 | (+)-TCAAACCAGACCCACCGAA, (−)-GCTGACCTCCACCTGCTGA, TETd-AACCTGCAGCTGAAGCCATCAGTGAATT-TAMRA |
| TGF-β | (+)-TGTGTGCGGCAGCTCTACAT, (−)-AGTTGGCATGGTAGCCCTTG, TET-ACTTCCGCAAGGACCTAGGATGGGAGT-TAMRA |
(+), forward primer; (−), reverse primer; FAM- or TET-TAMRA, probe.
FAM, 6-carboxyfluorescein.
TAMRA, 6-carboxy tetramethylrhodamine.
TET, 6-carboxy-4,7,2′,7′-tetrachlorofluorescein.
Construction and production of RNA standard templates for quantitative determination.
To prepare large amounts of RNA standard transcript for guinea pig IL-10, we first designed a DNA template for this cytokine: the T7 RNA polymerase promoter was linked to the 3′ end of the target DNA sequence specifically amplified with the two primers shown in Table 1. This template, synthesized by Hokkaido System Science Co., Ltd., was used to create the RNA transcript with RiboMax large-scale RNA production system T7 (Promega Corporation, Madison, Wis.). After digestion with RNase-free DNase, the resulting transcript was purified with Tris-EDTA-saturated phenol, chloroform, and isoamyl alcohol. The concentration of the RNA standard was determined by measuring optical density, and then the RNA was adjusted to an appropriate copy number with RNase-free water. The standard RNA dilutions were stored at −80°C. RNA standard transcripts for guinea pig IL-12, TGF-β, and IFN-γ were also synthesized by the same protocol.
Amplification of guinea pig cytokines by RT reaction and fluorogenic PCR.
To examine whether guinea pig rRNA could be quantitatively detected by using TaqMan rRNA control reagent, which consists of an rRNA probe labeled with VIC dye, primers, and control RNA (human) (Applied Biosystems), RT-PCR was carried out with a serial fourfold dilution of an RNA sample extracted from guinea pig PBMCs in triplicate. The RT-PCR was performed according to the manual of the TaqMan EZ RT-PCR kit (Applied Biosystems). The reaction mixture (total volume, 25 μl) consisted of 3 μl of appropriately diluted RNA sample; 5 μl of 5× TaqMan EZ buffer; 3 μl of 25 mM manganese acetate; 0.75 μl each of dATP, dCTP, dGTP, and dUTP; 0.25 μl of each primer at 100 μM; 1 μl of fluorogenic probe; 2.5 U of recombinant Tth DNA polymerase; 0.25 U of AmpErase uracil-N-glycosylase and 8.25 μl of RNase-free water. Thermal cycling conditions consisted of 2 min at 50°C, 30 min at 60°C, and 5 min at 95°C, followed by 50 cycles of 10 s at 95°C and 45 s at 62°C. The ABI Prism 7700 sequence detection system (Perkin-Elmer, Applied Biosystems Inc.) was employed for PCR cycling, real-time data collection, and analysis.
The level of gene expression was normalized with the amount of rRNA in the same RNA sample, and then data were shown in relative units as the ratios of the levels obtained in the immunized animals to those in the nonimmunized controls; ratios <1 imply down-regulation, and ratios >1 imply up-regulation.
DTH skin testing.
To investigate delayed-type hypersensitivity (DTH) skin reactions, 0.5 μg of PPD of tuberculin was injected intradermally into the BCG-immunized guinea pigs. Saline was used as a negative control. After 24 h, skin reactions were evaluated.
Statistical analysis.
Data analysis was carried out with the Statistica program (StatSoft, Tulsa, Okla.), and P values <0.05 were considered significant. A two-way analysis of variance (ANOVA) for repeated measures was used to evaluate the statistical significance of differences in levels of cytokine mRNA expression in PBMCs between the i.d. immunized and the i.v. immunized groups, followed by the Tukey test for pair-wise comparison when the ANOVA showed a significant difference. Levels of cytokine responses in spleens and lungs and the magnitudes of DTH responses for the two immunized groups were compared with the Mann-Whitney U test and the unpaired t test, respectively.
RESULTS
Precision of real-time RT-PCR by using the synthesized standard RNA templates.
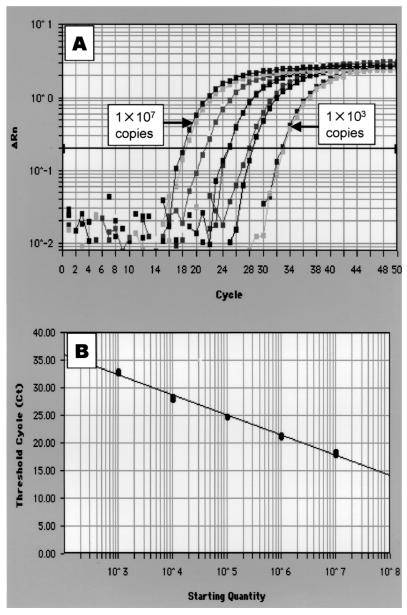
To quantify the expression of guinea pig cytokine genes, we synthesized standard RNA templates for IL-10, IL-12, TGF-β, and IFN-γ. When the IL-12 template serially diluted 10-fold in RNase-free water was amplified with the IL-12 primer-probe set by real-time RT-PCR, the amplification was dependent on the concentration of the RNA template (Fig. 1A), and the resulting standard curve showed linearity (r2 = 0.99) (Fig. 1B). Similar results were obtained with the IL-10, TGF-β, and IFN-γ templates (data not shown), indicating that the primers, the fluorescence-labeled probes, and the standard templates prepared in the present study were suitable for quantifying the expression of mRNA of cytokines in guinea pigs by real-time RT-PCR.
FIG. 1.
Real-time RT-PCR to quantify cytokine levels of guinea pigs. (A) PCR amplification of the standard RNA template for guinea pig IL-12 over 5 orders of magnitude (serial dilution of 107 to 103 copies) in duplicate by the ABI Prism 7700. The graph shows PCR cycle number versus difference in fluorescence (ΔRn). (B) Resulting standard curve for guinea pig IL-12. The standard curve was expressed as the starting quantity of target template versus threshold cycle (Ct). Ct is the PCR cycle at which a significant increase in ΔRn is first detected. Linearity was obtained over 5 orders of magnitude.
Detection of guinea pig rRNA by using the primer-probe set for human rRNA.
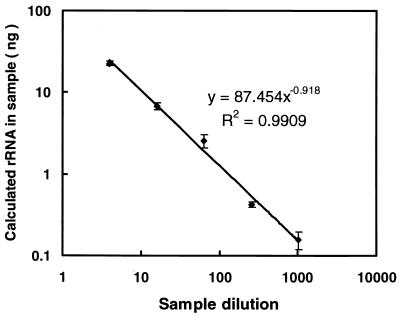
We attempted to amplify guinea pig rRNA with TaqMan rRNA control reagents because the rRNA has yet to be sequenced. When RNA extract from guinea pig PBMCs, diluted fourfold in RNase-free water, was amplified by real-time RT-PCR, the resulting amplification was highly dependent on rates of serial dilution over 5 orders of magnitude (r2 = 0.99) (Fig. 2). Therefore, we decided to use commercial RNA control reagents for detecting guinea pig rRNA as an internal control to normalize the RNA concentration in each sample.
FIG. 2.
Quantification of rRNA in serial fourfold dilutions of RNA samples extracted from guinea pig PBMCs by using TaqMan rRNA control reagents. The RT-PCR was run in triplicate for each sample. Shown are means ± standard deviations. Linearity was observed over 5 orders of magnitude, indicating that the amount of rRNA calculated with this reagent correctly reflects the concentration of each RNA sample solution.
Detection of cytokine mRNA in PBMCs from BCG-immunized guinea pigs.
To test whether the primer-probe sets for IL-10, IL-12, TGF-β, and IFN-γ can detect their target sequences in RNA derived from guinea pig PBMCs, we used animals immunized with either 0.1 mg of BCG vaccine by the i.d. route (i.d. immunized group) or 10 mg of BCG vaccine by the i.v. route (i.v. immunized group). The former vaccination is used to inoculate humans against tuberculosis.
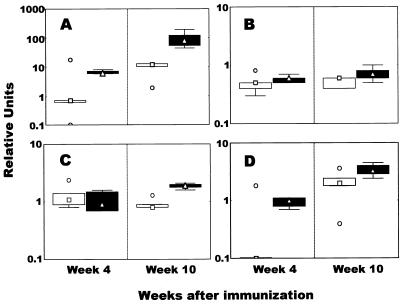
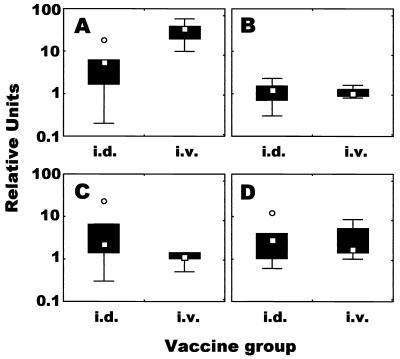
In the i.d. immunized group, IFN-γ mRNA levels in PBMCs were hardly increased at 4 weeks after immunization, while they were markedly increased at 10 weeks; however, the difference was not significant because of outliers in week 4 and week 10 (Fig. 3A). However, in the i.v. immunized group, mRNA for IFN-γ was highly expressed in PBMCs at both week 4 and week 10 (P = 0.001) (Fig. 3A).
FIG. 3.
Profile of mycobacterium-specific cytokine responses induced by BCG vaccination. PBMCs harvested 4 and 10 weeks after vaccination were stimulated in vitro with PPD of tuberculin antigen for 4 days. Total RNA was extracted, and IFN- γ (A), IL-12 (B), IL-10 (C), and TGF-β (D) mRNA levels were measured quantitatively by real-time RT-PCR. The results were expressed as relative units, as described in Materials and Methods. Shown are the medians (symbols in boxes; □, i.d. immunized group; Δ, i.v. immunized group), 25 to 75% response ranges (top and bottom lines of boxes), and nonoutlier minimums and maximums (whiskers). ○, values >1.5 box lengths.
Concerning IL-12, in the two immunized groups, there was no change in the level of mRNA expression compared to the baseline obtained from the nonimmunized group at both weeks 4 and 10 (Fig. 3B). Similar results for IL-10 mRNA expression in the i.d. immunized group were observed at weeks 4 and 10, while the expression in the i.v. immunized group was moderately elevated at week 10, and the difference between levels of expression in the i.v. immunized group at weeks 4 and 10 was significant (P = 0.036).
Although the relative level of TGF-β mRNA at week 4 in the i.d. immunized group was 10-fold lower than the baseline level (Fig. 3D), the expression in both immunized groups was significantly up-regulated at week 10 (Fig. 3D).
Detection of cytokine mRNA in spleen and BAL cells from BCG-immunized guinea pigs.
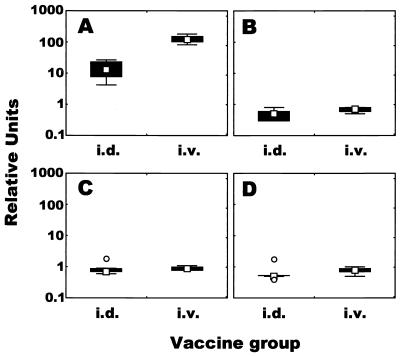
We injected PPD of tuberculin antigen into all of the guinea pigs intradermally at 8 weeks following the BCG vaccination and, 2 weeks later, sacrificed the animals to prepare spleen and BAL cells. In spleen cells, IFN-γ mRNA was expressed strongly in both immunized groups (Fig. 4A). The magnitude of IFN-γ mRNA expression in the i.v. immunized group was approximately 12-fold greater (median value) than that in the i.d. immunized group (P = 0.009). In contrast to IFN-γ mRNA expression, IL-10, IL-12, and TGF-β mRNA expression in both immunized groups was slightly reduced compared to the baseline expression (each median value was <1.0) (Fig. 4B to D). Likewise, in BAL cells, IFN-γ mRNA expression in the i.d. and i.v. immunized groups was up-regulated and the difference was significant (P = 0.016) (Fig. 5A). Concerning the levels of IL-10, IL-12, and TGF-β mRNA in BAL cells, little difference in relative units between the two groups was seen (Fig. 5B to D). However, there was considerable variability in the levels of mRNA of these cytokines in BAL cells; for example, the range in relative units of IL-10 mRNA expression in the i.d. immunized group was 0.3 to 22.6, indicating that the levels in BAL cells from some immunized animals were increased but that the levels in BAL cells from others were decreased.
FIG. 4.
Profile of mycobacterium-specific cytokine responses induced by BCG vaccination. Spleen cells harvested 10 weeks after vaccination were stimulated in vitro with PPD of tuberculin antigen for 4 days. Total RNA was extracted, and IFN- γ (A), IL-12 (B), IL-10 (C), and TGF-β (D) mRNA levels were measured quantitatively by real-time RT-PCR. The results were expressed as relative units, as described in Materials and Methods. Shown are the medians (symbols in boxes), 25 to 75% response ranges (top and bottom lines of boxes), and nonoutlier minimums and maximums (whiskers). ○, values >1.5 box lengths.
FIG. 5.
Profile of mycobacterium-specific cytokine responses induced by BCG vaccination. BAL cells harvested 10 weeks after vaccination were stimulated in vitro with PPD of tuberculin antigen for 4 days. Total RNA was extracted, and IFN- γ (A), IL-12 (B), IL-10 (C), and TGF-β (D) mRNA levels were measured quantitatively by real-time RT-PCR. The results were expressed as relative units, as described in Materials and Methods. Shown are the medians (symbols in boxes), 25 to 75% response ranges (top and bottom lines of boxes), and nonoutlier minimums and maximums (whiskers). ○, values >1.5 box lengths.
DTH skin reaction in response to PPD of tuberculin antigen.
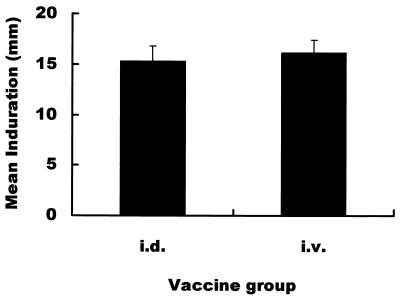
To investigate the correlation between the level of IFN-γ mRNA expression and the magnitude of the DTH skin reaction, we intradermally injected PPD of tuberculin antigen into all guinea pigs 8 weeks after the immunization with BCG. As shown in Fig. 6, there was no significant difference in the magnitude of PPD of tuberculin-specific induration between the i.d. immunized group and the i.v. immunized group.
FIG. 6.
DTH skin reaction specific for PPD of tuberculin antigen in the BCG-immunized guinea pigs. PPD of tuberculin antigen (0.5 μg) was i.d. injected into the immunized animals, and, 24 h later, the diameters of indurations were measured. Shown are means ± standard deviations.
DISCUSSION
The present study compared the dynamics of expression of cytokine mRNA in guinea pigs vaccinated with BCG with that in hyperimmunized animals inoculated i.v. by establishing a method for determining levels of cytokine mRNA in this useful animal model of infectious diseases (1, 8, 25, 36). It was found that IFN-γ mRNA levels in the vaccinated guinea pigs were 10 times higher than those in normal healthy controls at 10 weeks postinoculation. However, hyperimmunization with 10 mg of BCG i.v. increased the expression 100-fold compared to that for the normal controls, suggesting that extraordinarily high levels of IFN-γ mRNA expression are associated with BCG hyperimmunization which has been shown to accelerate the infectivity of human immunodeficiency virus type 1 and M. tuberculosis and progression to the diseased state (7, 43, 45).
To detect the expression of IL-10, IL-12, TGF-β, and IFN-γ genes quantitatively by the RT-PCR assay, the animals were immunized with BCG-Tokyo at a dose of 0.1 mg i.d. or 10 mg i.v. IL-12 is a potent inducer of Th1 cellular immune responses and is critical in providing resistance to intracellular pathogens (40). However, in the present study of BCG infection, no increase in IL-12 mRNA expression was found in lymphocytes derived from circulating blood, spleens, and lungs of the two immunized groups when these cells had been stimulated in vitro with PPD of tuberculin antigen for 4 days. One possible reason for this might be the much longer period of stimulation, because we confirmed a 20 to 50% increase in IL-12 mRNA expression in guinea pig PBMCs stimulated with concanavalin A (10 μg/ml) for 4 h, compared with that in nonstimulated PBMCs (data not shown). There were small changes in the levels of IL-10 and TGF-β gene expression in PBMCs. IL-10 mRNA levels were shown to be selectively increased at the site of disease in tuberculosis (2), and TGF-β was also shown to be more highly expressed in blood monocytes and granulomatous lung lesions from patients with active tuberculosis than in blood monocytes from healthy individuals (39) and exerts immunosuppressive activity against tuberculosis (12, 20, 21, 41). In the present study, we speculate that levels of IL-10 and TGF-β at 10 weeks following BCG administration, especially in the i.v. immunized group, increase to control the extremely strong IFN-γ response induced by high-dose BCG vaccination (14). However, no increase in the levels of IL-10 and TGF-β mRNA in spleen and BAL cells from either immunized group was observed. It is not clear why these responses occurred in PBMCs but not in spleen cells and BAL fluid. One possibility is that BCG inoculation does not induce activation of IL-10 and TGF-β in spleen and BAL cells and that increases in the expression of mRNA of these cytokines may be closely associated with the pathogenicity of the invader (3). Our findings could be attributed to the differences in cytokine responses against BCG infection in PBMCs, splenocytes, and BAL cells from immunized guinea pigs. It has been reported that no changes in the production of IL-10 and TGF-β in humans who had received a BCG vaccination by the oral route were seen (23). On the other hand, in BAL cells from acute-phase tuberculosis patients, it has been shown that IL-1β, IL-6, IL-8, tumor necrosis factor alpha, monocyte chemoattractant protein 1, and RANTES levels are markedly elevated (29, 30) but decrease in the convalescent phase (29), suggesting that, in addition to pathogenicity, the stage of disease may be closely associated with cytokine responses. It is possible that the mechanism regulating cytokine production in BCG administration differs from that during a virulent mycobacterial infection. In contrast to IL-10 and TGF-β, IFN-γ, which has been shown to be crucial for the control of cell-associated pathogens such as M. tuberculosis (11, 16, 38), was strongly expressed in PBMCs, spleen, and BAL cells in our study. In PBMCs stimulated with PPD of tuberculin antigen in vitro, the level of IFN-γ gene expression in the i.v. immunized group, but not in the i.d. immunized group, was greatly increased at week 4, whereas, at week 10, both groups clearly showed an increase. These findings indicate that a Th1-type immune response against BCG might be raised gradually over a 10-week period after immunization and/or that an antigen-specific memory T-cell population might be expanded and then might preferentially specialize for Th1-type effector cells by the injection of PPD of tuberculin antigen for DTH skin testing at week 8. This might result in a greater increase in IFN-γ mRNA at week 10 than at week 4. On comparing the i.v. immunized group with the i.d. immunized group, we found that the higher dose of BCG invariably induced a higher level of expression of IFN-γ mRNA in PBMCs, spleen, and BAL cells. In the spleen, the relative level of cytokine expression per 107 splenocytes can be converted to the absolute level. The mean total number of splenocytes was sixfold higher in the former group than in the latter group at 10 weeks after BCG immunization. This means that the level of IFN-γ mRNA expression in whole spleen in the i.v. immunized group was approximately 60-fold greater than that in the i.d. immunized group because the relative level is 10-fold higher in the former than in the latter. On the other hand, no change in the mean total number of PBMCs and BAL cells was observed in either group. It has been reported that a low-dose vaccination preferentially induces a Th1-predominant immunity, while a high dose develops predominantly a Th2-type response, as indicated by a study on the vaccination of mice with Leishmania major (5). However, we do not interpret our results as reflecting an induction of Th1-predominant immunity by high-dose BCG vaccination, which may lead to chronic infection rather than immunity, during the period of the experiment (4). In short, it is possible that the cell-mediated immune reaction triggered by 10 mg of BCG administered i.v. responded aggressively to the infection during the period of the experiment. It should be noted that 0.1 mg of BCG i.d., the dose used to vaccinate humans, preferentially developed a Th1-type response in circulating blood, spleens, and lungs from immunized guinea pigs. Recently, Jeevan et al. have shown that IL-1β and RANTES mRNA expression in splenocytes from guinea pigs immunized intradermally with BCG is up-regulated when these cells are stimulated in vitro with PPD of tuberculin (26). IL-1β has been shown to play an important role in host resistance to mycobacteria (44), and RANTES was shown to induce migration of macrophages in guinea pigs (6) and of blood monocytes and memory T cells in humans (35). The efficacy with which BCG vaccination is able to significantly elevate the levels of IL-1β, RANTES (26), and IFN-γ expression could contribute to the effective prevention of infant tuberculosis.
It is interesting that the levels of IFN-γ mRNA expression in PBMCs and spleen cells in the i.v. immunized group were much higher than those in the i.d. immunized group; nevertheless there was no significant difference in the magnitude of the DTH response to PPD of tuberculin antigen between these groups. This result indicates that the response by IFN-γ in systemic compartments does not necessarily reflect the DTH skin response: higher levels of IFN-γ are not always associated with a stronger skin response. Similarly, it was reported that orally vaccinated humans did not exhibit a DTH skin response to tuberculin but produced tuberculin-specific IFN-γ (23), suggesting that different T-cell subpopulations are responsible for DTH and IFN-γ production (27). In this study, it is possible that i.v. inoculation of guinea pigs with 10 mg of BCG preferentially induced the expansion of a T-cell subpopulation related to the IFN-γ response. Our findings show that administration of a large amount of BCG engenders a strong IFN-γ response. Other studies using a mouse model have also shown that increased IFN-γ production does not lead to enhanced protective efficacy against M. tuberculosis infection (31) and that the protective efficacy might involve an optimal amount of IFN-γ and its overexpression might be superfluous (31). The hyperactivity of T cells in response to BCG, characterized by extremely high levels of IFN-γ, may be accompanied by acceleration of M. tuberculosis infection or human immunodeficiency virus type 1 coinfection (7, 43, 45). With respect to the correlation between sensitivity to tuberculin and protective immunity conferred by the BCG vaccine, a previous report showed that subjects whose DTH responses were much stronger than average had an enhanced incidence of tuberculosis compared with those with weak or moderate responses (15). A similar concept, that protection from tuberculosis by the BCG vaccine did not depend on the degree of skin sensitivity to tuberculin, was also reported (10). Nevertheless, DTH skin testing is widespread, and a positive response is presently thought to indicate a successful vaccination. The test is easy to perform and is the only assay of cell-mediated immunity which can be performed on a large scale in the field. One must carefully assess whether immunity to tuberculosis is conferred by BCG, because it is possible that individuals who have actually acquired immunity but who do not show a DTH response could be vaccinated again if the presence of acquired immunity is judged only from the results of DTH skin testing. Any additional vaccination could lead to an undesirable shift in the immunological balance. Therefore, it is important to investigate the association between protection against tuberculosis, level of DTH skin response, and IFN-γ production in more detail.
We applied a real-time quantitative RT-PCR to monitor the kinetics of cytokine mRNA expression. This assay has recently been used for detecting cytokine mRNA expression in mice (18, 19) and rhesus macaques (22), and it has been demonstrated that real-time RT-PCR is the most sensitive assay to quantitate cytokine expression reproducibly. Interestingly, we could quantitatively measure basal levels of cytokine mRNA expression in PBMCs, splenocytes, and BAL cells from nonimmunized guinea pigs, indicating that real-time RT-PCR is well suited to exactly detect the change in the level of cytokine gene expression induced by vaccination. A previous report showed that a considerable level of IFN-γ was expressed in splenocytes from nonimmunized guinea pigs by using the traditional agarose gel RT-PCR method; this finding led to the result that the difference in the level of IFN-γ expression between BCG-immunized and nonimmunized animals was impossible to quantify by detection with densitometry (28). Having a cytokine mRNA detection range of 5 log units, our assay system can quantitate the markedly increased IFN-γ expression caused by BCG vaccination. Furthermore, it is possible to monitor the kinetics of different types of dyes concurrently in one running of the ABI Prism 7700. As shown in Table 1 and in Materials and Methods, we chose three types of dyes to label the probes: FAM (IL-10 and IFN-γ), TET (IL-12 and TGF-β), and VIC (rRNA); this contributed to an efficient analysis of a large amount of sample.
In conclusion, we have established an extremely useful assay by using real-time RT-PCR to quantitatively measure guinea pig IFN-γ, IL-12, IL-10, and TGF-β mRNA and have found an augmentation of IFN-γ mRNA expression related to DTH induction in guinea pigs immunized i.d. with 0.1 mg of BCG. On the other hand, extremely large amounts of IFN-γ were produced in animals immunized i.v. with 10 mg of BCG, the levels not being correlated with the enhancement of the magnitude of DTH. Our findings suggest that there may be an intricate relationship between protective immunity, the level of IFN-γ response, and the magnitude of DTH. It will be worthwhile to investigate the optimal IFN-γ response related to the protective efficacy of BCG against M. tuberculosis infection.
Acknowledgments
We thank Michio Ohba of Kyushu University in Japan for helpful discussions.
This work was supported by funding from the Japan Health Sciences Foundation and the Ministry of Health, Labor and Welfare, Japan.
Editor: J. D. Clements
REFERENCES
- 1.Balasubramanian, V., E. Wiegeshaus, and D. W. Smith. 1994. Mycobacterial infection in guinea pigs. Immunobiology 191:395-401. [DOI] [PubMed] [Google Scholar]
- 2.Barnes, P. F., S. Lu, J. S. Abrams, E. Wang, M. Yamamura, and R. L. Modlin. 1993. Cytokine production at the site of disease in human tuberculosis. Infect. Immun. 61:3482-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bermudez, L. E. 1993. Production of transforming growth factor-β by Mycobacterium avium-infected human macrophages is associated with unresponsiveness to IFN-γ. J. Immunol. 150:1838-1845. [PubMed] [Google Scholar]
- 4.Bretscher, P. A. 1992. A strategy to improve the efficacy of vaccination against tuberculosis and leprosy. Immunol. Today 13:342-345. [DOI] [PubMed] [Google Scholar]
- 5.Bretscher, P. A., G. Wei, J. N. Menon, and H. Bielefeldt-Ohmann. 1992. Establishment of stable, cell-mediated immunity that makes “susceptible” mice resistant to Leishmania major. Science 257:539-542. [DOI] [PubMed] [Google Scholar]
- 6.Campbell, E. M., A. E. I. Proudfood, T. Yoshimura, B. Allet, T. N. C. Wells, A.-M. White, J. Westwick, and M. L. Watson. 1997. Recombinant guinea pig and human RANTES activate macrophages but not eosinophils in the guinea pig. J. Immunol. 159:1482-1489. [PubMed] [Google Scholar]
- 7.Cheynier, R., S. Gratton, M. Halloran, I. Stahmer, N. L. Letvin, and S. Wain-Hobson. 1998. Antigenic stimulation by BCG vaccine as an in vivo driving force for SIV replication and dissemination. Nat. Med. 4:421-427. [DOI] [PubMed] [Google Scholar]
- 8.Cohen, M. K., R. A. Bartow, C. L. Mintzer, and D. N. McMurray. 1987. Effects of diet and genetics on Mycobacterium bovis BCG vaccine efficacy in inbred guinea pigs. Infect. Immun. 55:314-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohn, M. L., C. L. Davis, and G. Middlebrook. 1962. Chemoprophylaxis with isoniazid against aerogenic tuberculosis infection in the guinea pig. Am. Rev. Respir. Dis. 86:95-97. [DOI] [PubMed] [Google Scholar]
- 10.Comstock, G. W. 1988. Identification of an effective vaccine against tuberculosis. Am. Rev. Respir. Dis. 138:479-480. [DOI] [PubMed] [Google Scholar]
- 11.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon γ gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai, G., and D. N. McMurray. 1999. Effects of modulating TGF-beta 1 on immune responses to mycobacterial infection in guinea pigs. Tuber. Lung. Dis. 79:207-214. [DOI] [PubMed] [Google Scholar]
- 13.Dascher, C. C., K. Hiromatsu, J. W. Naylor, P. P. Brauer, K. A. Brown, J. R. Storey, S. M. Behar, E. S. Kawasaki, S. A. Porcelli, M. B. Brenner, and K. P. LeClair. 1999. Conservation of a CD1 multigene family in the guinea pig. J. Immunol. 163:5478-5488. [PubMed] [Google Scholar]
- 14.Espevik, T., I. S. Figari, M. R. Shalaby, G. A. Lackides, G. D. Lewis, H. M. Shepard, and M. A. Palladino, Jr. 1987. Inhibition of cytokine production by cyclosporin a and transforming growth factor β. J. Exp. Med. 166:571-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fine, P. E., J. A. Sterne, J. M. Ponnighaus, and R. J. Rees. 1994. Delayed-type hypersensitivity, mycobacterial vaccines and protective immunity. Lancet 344:1245-1249. [DOI] [PubMed] [Google Scholar]
- 16.Flynn, J.-A. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon γ in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forster, V. T. 1948. Zwischenmolekulare Energiewanderung und fluoreszenz. Ann. Phys. 2:55-75. [Google Scholar]
- 18.Hein, J., U. Schellenberg, G. Bein, and H. Hackstein. 2001. Quantification of murine IFN-γ mRNA and protein expression: impact of real-time kinetic RT-PCR using SYBR green I dye. Scand. J. Immunol. 54:285-291. [DOI] [PubMed] [Google Scholar]
- 19.Hempel, D. M., K. A. Smith, K. A. Claussen, and M. A. Perricone. 2002. Analysis of cellular immune responses in the peripheral blood of mice using real-time RT-PCR. J. Immunol. Methods 259:129-138. [DOI] [PubMed] [Google Scholar]
- 20.Hirsch, C. S., R. Hussain, Z. Toossi, G. Dawood, F. Shahid, and J. J. Ellner. 1996. Cross-modulation by transforming growth factor β in human tuberculosis: suppression of antigen-driven blastogenesis and interferon γ production. Proc. Natl. Acad. Sci. USA 93:3193-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirsch, C. S., T. Yoneda, L. Averill, J. J. Ellner, and Z. Toossi. 1994. Enhancement of intracellular growth of Mycobacterium tuberculosis in human monocytes by transforming growth factor-β1. J. Infect. Dis. 170:1229-1237. [DOI] [PubMed] [Google Scholar]
- 22.Hofmann-Lehmann, R., A. L. Williams, R. K. Swenerton, P.-L. Li, R. A. Rasmussen, A.-L. Chenine, H. M. McClure, and R. M. Ruprecht. 2002. Quantitation of simian cytokine and β-chemokine mRNAs, using real-time reverse transcriptase-polymerase chain reaction: variations in expression during chronic primate lentivirus infection. AIDS Res. Hum. Retroviruses 18:627-639. [DOI] [PubMed] [Google Scholar]
- 23.Hoft, D. F., R. M. Brown, and R. B. Belshe. 2000. Mucosal bacille Calmette-Guerin vaccination of humans inhibits delayed-type hypersensitivity to purified protein derivative but induces mycobacteria-specific interferon-γ responses. Clin. Infect. Dis. 30:S217-S222. [DOI] [PubMed] [Google Scholar]
- 24.Holland, P. M., R. D. Abramson, R. Watson, and D. H. Gelfand. 1991. Detection of specific polymerase chain reaction product by utilizing the 5′→3′ exonuclease activity of Thermus aquaticus DNA polymerase. Proc. Natl. Acad. Sci. USA 88:7276-7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson, M., S. W. Phalen, M. Lagranderie, D. Ensergueix, P. Chavarot, G. Marchal, D. N. McMurray, B. Gicquel, and C. Guilhot. 1999. Persistence and protective efficacy of a Mycobacterium tuberculosis auxotroph vaccine. Infect. Immun. 67:2867-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeevan, A., T. Yoshimura, G. Foster, and D. N. McMurray. 2002. Effect of Mycobacterium bovis BCG vaccination on interleukin-1β and RANTES mRNA expression in guinea pig cells exposed to attenuated and virulent mycobacteria. Infect. Immun. 70:1245-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawamura, I., H. Tsukada, H. Yoshikawa, M. Fujita, K. Nomoto, and M. Mitsuyama. 1992. IFN-γ-producing ability as a possible marker for the protective T cells against Mycobacterium bovis BCG in mice. J. Immunol. 148:2887-2893. [PubMed] [Google Scholar]
- 28.Klünner, T., T. Bartels, M. Vordermeier, R. Burger, and H. Schäfer. 2001. Immune reactions of CD4- and CD8-positive T cell subpopulations in spleen and lymph nodes of guinea pigs after vaccination with bacillus Calmette-Guerin. Vaccine 19:1968-1977. [DOI] [PubMed] [Google Scholar]
- 29.Kurashima, K., N. Mukaida, M. Fujimura, M. Yasui, Y. Nakazumi, T. Matsuda, and K. Matsushima. 1997. Elevated chemokine levels in bronchoalveolar lavage fluid of tuberculosis patients. Am. J. Crit. Care Med. 155:1474-1477. [DOI] [PubMed] [Google Scholar]
- 30.Law, K., M. Weiden, T. Harkin, K. Tchou-Wong, C. Chi, and W. N. Rom. 1996. Increased release of interleukin-1β, interleukin-6, and tumor necrosis factor-α by bronchoalveolar cells lavaged from involved sites in pulmonary tuberculosis. Am. J. Crit. Care Med. 153:799-804. [DOI] [PubMed] [Google Scholar]
- 31.Leal, I. S., B. Smedegard, P. Andersen, and R. Appelberg. 2001. Failure to induce enhanced protection against tuberculosis by increasing T-cell-dependent interferon-γ generation. Immunology 104:157-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyamichev, V., M. A. Brow, and J. E. Dahlberg. 1993. Structure-specific endonucleolytic cleavage of nucleic acids by eubacterial DNA polymerases. Science 260:778-783. [DOI] [PubMed] [Google Scholar]
- 33.Nathan, C. F., H. W. Murray, M. E. Wiebe, and B. Y. Rubin. 1983. Identification of interferon-γ as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J. Exp. Med. 158:670-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scarozza, A. M., A. I. Ramsingh, V. Wicher, and K. Wicher. 1998. Spontaneous cytokine gene expression in normal guinea pig blood and tissues. Cytokine 10:851-859. [DOI] [PubMed] [Google Scholar]
- 35.Schall, T. J., K. Bacon, K. J. Toy, and D. V. Goeddel. 1990. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature 347:669-671. [DOI] [PubMed] [Google Scholar]
- 36.Smith, D. W., D. N. McMurray, E. H. Wiegeshaus, A. A. Grover, and G. E. Harding. 1970. Host-parasite relationships in experimental airborne tuberculosis. Am. Rev. Respir. Dis. 102:937-949. [DOI] [PubMed] [Google Scholar]
- 37.Smith, D. W., V. Balasubramanian, and E. Wiegeshaus. 1991. A guinea pig model of experimental airborne tuberculosis for evaluation of the response to chemotherapy: the effect on bacilli in the initial phase of treatment. Tubercle 72:223-231. [DOI] [PubMed] [Google Scholar]
- 38.Tascon, R. E., E. Stravropoulos, K. V. Lukacs, and M. J. Colston. 1998. Protection against Mycobacterium tuberculosis infection by CD8+ T cells requires the production of gamma interferon. Infect. Immun. 66:830-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toossi, Z., P. Gogate, H. Shiratsuchi, T. Young, and J. J. Ellner. 1995. Enhanced production of TGF-beta by blood monocytes from patients with active tuberculosis and presence of TGF-beta in tuberculous granulomatous lung lesions. J. Immunol. 154:465-473. [PubMed] [Google Scholar]
- 40.Trinchieri, G. 1994. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood 84:4008-4027. [PubMed] [Google Scholar]
- 41.Tsunawaki, S., M. Sporn, A. Ding, and C. Nathan. 1988. Deactivation of macrophages by transforming growth factor-β. Nature 334:260-262. [DOI] [PubMed] [Google Scholar]
- 42.Ulrichs, T., and S. A. Porcelli. 2000. CD1 proteins: targets of T cell recognition in innate and adaptive immunity. Rev. Immunogenet. 2:416-432. [PubMed] [Google Scholar]
- 43.Zhang, Y., K. Nakata, M. Weiden, and W. M. Rom. 1995. Mycobacterium tuberculosis enhances human immunodeficiency virus-1 replication by transcriptional activation at the long terminal repeat. J. Clin. Investig. 95:2324-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang, Y., M. Doerfler, T. C. Lee, B. Guillemin, and W. N. Rom. 1993. Mechanisms of stimulation of interleukin-1β and tumor necrosis factor-α by Mycobacterium tuberculosis components. J. Clin. Investig. 91:2076-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou, D., Y. Shen, L. Chalifoux, D. Lee-Parritz, M. Simon, P. K. Sehgal, L. Zheng, M. Halloran, and Z. W. Chen. 1999. Mycobacterium bovis bacille Calmette-Guérin enhances pathogenicity of simian immunodeficiency virus infection and accelerates progression to AIDS in macaques: a role of persistent T cell activation in AIDS pathogenesis. J. Immunol. 162:2204-2216. [PubMed] [Google Scholar]