Abstract
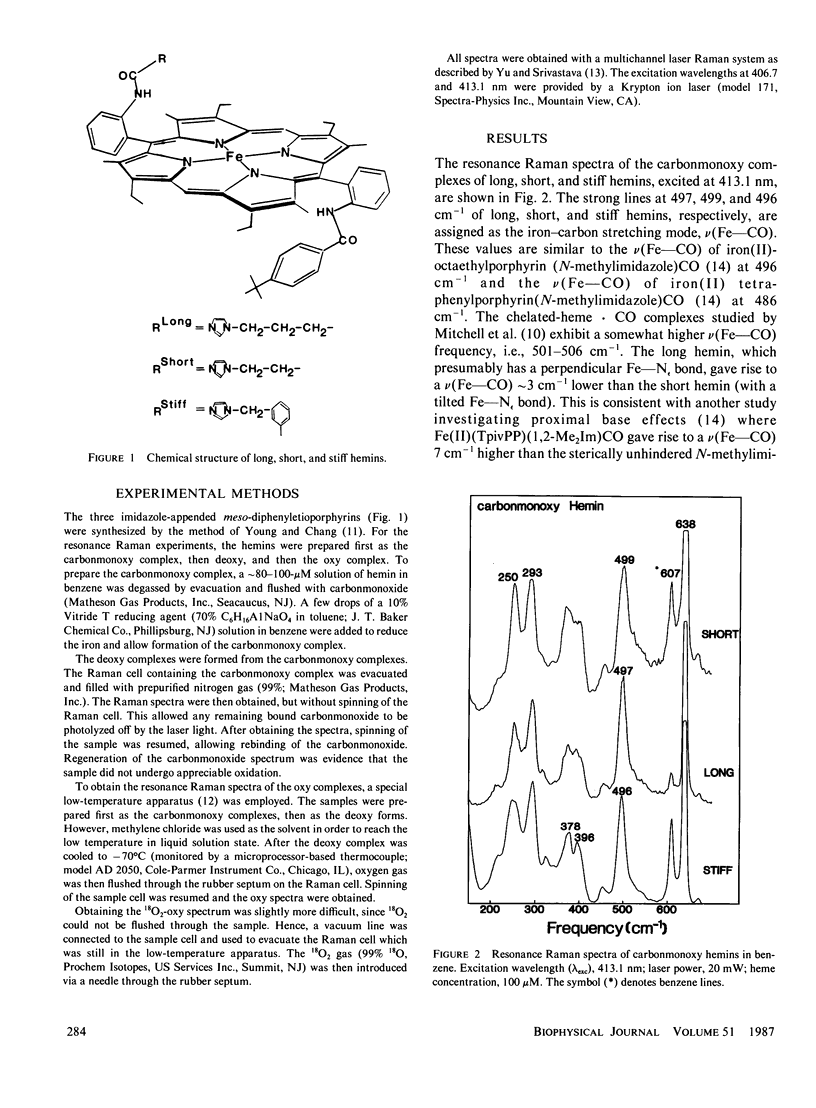
Resonance Raman spectroscopy has been employed to probe the effects of proximal base strain on the bonding of O2 and CO in three synthetic hemins with covalently linked imidazole ligands. The strain is introduced by varying the length of the imidazole-containing side chain and by restricting the side chain flexibility with a phenyl ring. These hemins are abbreviated as "long," "short," and "stiff" hemins, respectively. In the deoxy state, the iron-imidazole stretching frequencies [nu(Fe--N epsilon)] for long, short, and stiff hemins are detected at 200, 207, and 204 cm-1, respectively. The strain induced in the iron-imidazole bond by the short hemin results in a higher nu(Fe--N epsilon) frequency, in contrast to the strain induced by sterically hindered 2-methylimidazole or 1,2-dimethylimidazole complexes in which the Fe--N epsilon bond is tilted and lengthened, but the imidazole ring remains perpendicular to the heme plane. However, in the short hemin, the plane of the imidazole ring may not be perpendicular to the plane of the porphyrin, altering the amount of pi-interaction (hence the strength of Fe--N epsilon bond) and the nature of normal mode containing Fe--N epsilon bond stretching. Upon CO binding, we have observed the nu(Fe--CO) stretching frequencies at 497 (long), 499 (short), and 496 cm-1 (stiff), somewhat lower than those reported by Mitchell et al. (Inorg. Chem., 1985, 24:967) for the chelated-heme X CO complexes (i.e., 501-506 cm-1). This is the first report of an iron-oxygen-associated vibration observed in solution for an unprotected heme. The oxy complexes were formed by introducing dioxygen to the deoxy complexes at -700C. The isotope-sensitive line was detected at 576 cm- (1602) in oxy stiff hemin, which was shifted to 545 cm-1 upon 1802 substitution. This is perhaps the largest isotope shift (31 cm-') observed to date, compared with the usual 22-24 cm-'.For the long and short hemins, the iron-oxygen-associated vibration was detected at 574 and 573 cm-', respectively.These values are very similar to those observed(N-methylimidazole) and myoglobin/hemoglobin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benko B., Yu N. T. Resonance Raman studies of nitric oxide binding to ferric and ferrous hemoproteins: detection of Fe(III)--NO stretching, Fe(III)--N--O bending, and Fe(II)--N--O bending vibrations. Proc Natl Acad Sci U S A. 1983 Nov;80(22):7042–7046. doi: 10.1073/pnas.80.22.7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. K., Traylor T. G. Solution behavior of a synthetic myoglobin active site. J Am Chem Soc. 1973 Aug 22;95(17):5810–5811. doi: 10.1021/ja00798a088. [DOI] [PubMed] [Google Scholar]
- Cohen I. A., Caughey W. S. Substituted deuteroporphyrins. IV. On the kinetics and mechanism of reactions of iron(II) porphyrins with oxygen. Biochemistry. 1968 Feb;7(2):636–641. doi: 10.1021/bi00842a019. [DOI] [PubMed] [Google Scholar]
- Collman J. P., Gagne R. R., Halbert T. R., Marchon J. C., Reed C. A. Letter: Reversible oxygen adduct formation in ferrous complexes derived from a "picket fence" prophyrin. A model for oxymyoglobin. J Am Chem Soc. 1973 Nov 14;95(23):7868–7870. doi: 10.1021/ja00804a054. [DOI] [PubMed] [Google Scholar]
- Dickmann H., Chang C. K., Traylor T. G. Cyclophane porphyrin. J Am Chem Soc. 1971 Aug 11;93(16):4068–4070. doi: 10.1021/ja00745a053. [DOI] [PubMed] [Google Scholar]
- Kerr E. A., Mackin H. C., Yu N. T. Resonance Raman studies of carbon monoxide binding to iron "picket fence" porphyrin with unhindered and hindered axial bases. An inverse relationship between binding affinity and the strength of iron-carbon bond. Biochemistry. 1983 Sep 13;22(19):4373–4379. doi: 10.1021/bi00288a005. [DOI] [PubMed] [Google Scholar]
- Kincaid J., Stein P., Spiro T. G. Absence of heme-localized strain in T state hemoglobin: insensitivity of heme-imidazole resonance Raman frequencies to quaternary structure. Proc Natl Acad Sci U S A. 1979 Feb;76(2):549–552. doi: 10.1073/pnas.76.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackin H. C., Tsubaki M., Yu N. T. Resonance Raman studies of Co-O2 and O-O stretching vibrations in oxy-cobalt hemes. Biophys J. 1983 Mar;41(3):349–357. doi: 10.1016/S0006-3495(83)84446-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K., Kitagawa T., Morimoto H. Quaternary structures and low frequency molecular vibrations of haems of deoxy and oxyhaemoglobin studied by resonance raman scattering. J Mol Biol. 1980 Jan 25;136(3):271–289. doi: 10.1016/0022-2836(80)90374-5. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Regulation of oxygen affinity of hemoglobin: influence of structure of the globin on the heme iron. Annu Rev Biochem. 1979;48:327–386. doi: 10.1146/annurev.bi.48.070179.001551. [DOI] [PubMed] [Google Scholar]
- Tsubaki M., Srivastava R. B., Yu N. T. Resonance Raman investigation of carbon monoxide bonding in (carbon monoxy)hemoglobin and -myoglobin: detection of Fe-CO stretching and Fe-C-O bending vibrations and influence of the quaternary structure change. Biochemistry. 1982 Mar 16;21(6):1132–1140. doi: 10.1021/bi00535a004. [DOI] [PubMed] [Google Scholar]
- Yu N. T., Benko B., Kerr E. A., Gersonde K. Iron-carbon bond lengths in carbonmonoxy and cyanomet complexes of the monomeric hemoglobin III from Chironomus thummi thummi: a critical comparison between resonance Raman and x-ray diffraction studies. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5106–5110. doi: 10.1073/pnas.81.16.5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu N. T. Resonance Raman studies of ligand binding. Methods Enzymol. 1986;130:350–409. doi: 10.1016/0076-6879(86)30018-1. [DOI] [PubMed] [Google Scholar]


