Abstract
One of the more perplexing aspects of urinary tract infections (UTIs) is their high propensity to recur. It has been proposed that recurrent infections are a result of the reintroduction of bacteria from the gastrointestinal tract (GIT) to the urinary tract (UT); however, since a significant subset of recurrent UTIs are caused by an identical bacterial strain, it has been challenging to formally prove this hypothesis for same-strain recurrences by using epidemiologic approaches. We present data here obtained by using a mouse model of UTIs in which it was shown that 36% (5 of 14) of mice infected with uropathogenic Escherichia coli (UPEC) will have at least one bacteriuric recurrence, with 21% (3 of 14) having more than one recurrence during a 6-week period after an acute UTI. Intraurethrally infected mice develop UPEC reservoirs in both their feces and their bladders. Ten days of trimethoprim-sulfamethoxazole (SXT) therapy reduces urinary recurrences and eradicates fecal colonization, whereas 3 days of SXT treatment has no effect over a twenty-eight-day observation period despite clearing fecal colonization acutely. Interestingly, SXT is unable to eradicate bacteria from the bladder reservoir even after a 10-day treatment regimen, thus demonstrating that the bladder reservoir can persist even in the face of long-term antibiotic therapy.
Recurrent urinary tract infections (UTIs) are a common clinical problem. Up to 25% of women who present with an acute UTI will have a recurrence within 6 months, despite receiving appropriate antibiotic therapy (3). The majority of both acute and recurrent UTIs are caused by uropathogenic Escherichia coli (UPEC) (7). Interestingly, a significant number of recurrent UTIs are caused by the identical strain of E. coli isolated from the index UTI (1, 4, 10, 11, 18). Recurrences within the first month of infection are even more likely to be caused by the same strain of E. coli (10, 18). In some instances, infections caused by the same bacterial strain have been observed over a year after the original infection (23).
The high frequency of the same strain recurrences suggests the existence of a UPEC reservoir in close proximity to the affected individual. The current dogma is that recurrent UTIs result from the reinoculation of the UT with bacteria originating from the gastrointestinal tract (GIT) flora (6). Consistent with this idea, it has been demonstrated that the GIT and the vaginal-periurethral areas can be a reservoir for UPEC (18, 22). In addition, vaginal epithelial cells from women that are prone to recurrent UTIs tend to support higher levels of bacterial adherence compared to cells from healthy controls (20). However, the presence of perineal bacterial colonization, or GIT colonization, does not predict the risk of recurrence in girls or women with anatomically normal urinary tracts (UTs), arguing that there are additional factors involved in recurrent UTI pathogenesis (21, 25). Previous studies have also shown that the daily application of antibacterial ointment to the perineal area fails to prevent recurrent infections, even though such a treatment would be expected to block fecal to UT transmission (2). Such results suggest that the GIT and perineal areas may not be the only reservoir for UPEC.
The observation that bacteria are cleared from the urine following an acute UTI has led to the assumption that UPEC is eradicated from the urinary tract. However, this view has been challenged by a recent study demonstrating that mice infected with type 1-piliated UPEC are able to persist in the bladder for up to 6 weeks after acute infection in the absence of bacteriuria (8, 16). Thus, the bladder appears to be a previously unrecognized long-term reservoir for UPEC, which may contribute to the recurrent nature of UTIs. Virtually all uropathogenic E. coli express type 1 pili, which mediate binding to and invasion into uroplakin-coated bladder epithelial cells. The FimH-uroplakin interaction activates signal transduction pathways, leading to the invasion of the bacterium into the superficial umbrella cells, where they are protected from the flow of urine and other host defenses (9, 19). UPEC replicates rapidly once inside these cells, creating large foci of bacteria called “bug factories.” The host responds by initiating numerous innate defenses in the bladder. An apoptosis-like response is activated, leading to the exfoliation of the superficial umbrella cells under attack by the invading pathogen. Complex signaling pathways are also activated and result in the host inflammatory response. In order to persist in the bladder in the face of these host defenses, the UPEC bug factories “flux” or break out of umbrella cells and colonize the exposed underlying transitional epithelium as part of a process to invade deeper tissues and establish a chronic reservoir, which in mice can persist for months in a quiescent state (17).
The majority of acute UTIs respond well to antibiotic therapy with agents such as trimethoprim-sulfamethoxazole (SXT). The recommended treatment regimen for women with acute cystitis is a 3-day course of antibiotics; however, despite cure of the acute infections, as defined by clearance of bacteria from the urine, the rate of relapse or recurrence is as high as 15% within 6 weeks after the cessation of treatment (14). In addition, prophylactic antibiotic therapy is commonly used for the management of recurrent UTIs; however, if the antibiotics are discontinued, the infections tend to recur (24). In the present study, we sought to investigate the potential role of the bladder bacterial reservoir in recurrent bacteriuria and to determine how the duration of antibiotic therapy affects the natural history of bacterial persistence and recurrence.
MATERIALS AND METHODS
Bacteria and growth conditions.
UTI89 (16) and UTI89 SR (a streptomycin-resistant derivative of the same strain) were grown static for 48 h in Luria broth at 37°C to induce the expression of type 1 pili. UTI89 is SXT sensitive.
Mouse inoculations.
C57BL/6 female mice were obtained from Jackson Laboratory. A 48-h culture of UTI89 was pelleted and resuspended in sterile phosphate-buffered saline (PBS) at a concentration of ca. 2 × 109 CFU/ml. Eight- to twelve-week-old mice were infected via intraurethral inoculation with 50 μl of the bacterial suspension (108 CFU) (15). Anal lavages were performed by using 100 μl of the same bacterial suspension described above. At the indicated times after infection, mice were individually induced to urinate by applying gentle pressure to the skin just below the occiput. The clean-catch urine samples were collected into sterile Eppendorf tubes, and the bacterial titers were determined by plating serial dilutions of the material onto Luria-Bertani (LB) agar with or without streptomycin. Fecal samples were likewise collected individually and subsequently homogenized in 250 μl of LB medium. The homogenate was plated onto streptomycin-containing agar plates to determine the fecal titer. Care was taken to ensure that cross-contamination between urine and fecal samples did not occur. Bacterial titers in the bladder or kidney were determined by homogenizing the tissue in 500 μl or 1 ml of sterile PBS containing 0.025% Triton X-100, respectively, and plating out serial dilutions of the homogenate onto LB agar with or without streptomycin.
For the antibiotic therapy experiments, mice were treated for the indicated duration with SXT (Qualitest Pharmaceuticals, Huntsville, Ala.) at concentrations of 54 and 270 μg/ml, respectively, in their drinking water.
DNA fingerprinting.
The techniques and primer sets for the arbitrarily primed PCR fingerprinting were used as previously described (12).
RESULTS
Recurrent bacteriuria in mice infected with UPEC.
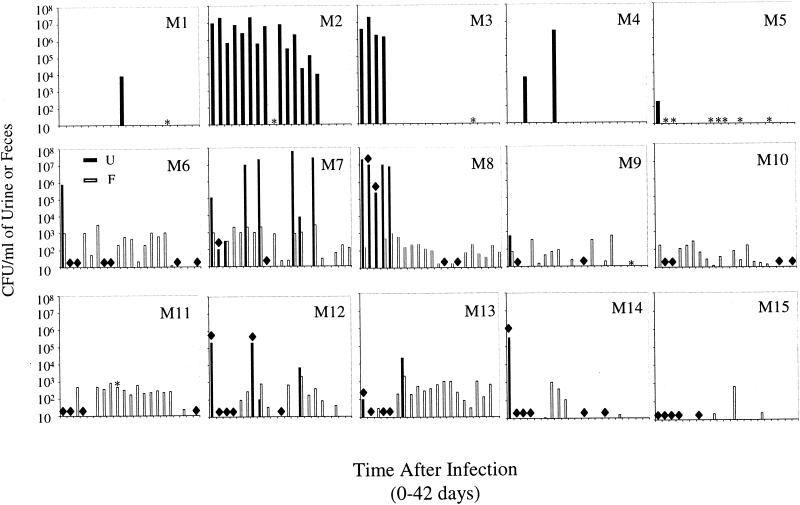
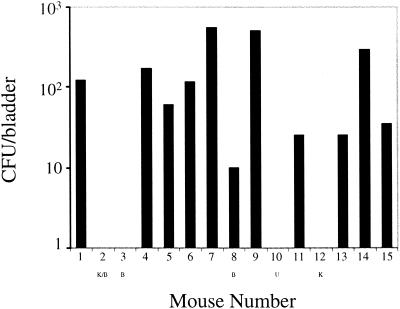
It has previously been observed that mice with reservoir levels of bacteria in the bladder (50 to 1,000 CFU/bladder) have sterile urine, whereas mice with acute infection levels of bacteria in the bladder (105 to 107 CFU/bladder) have high-titer bacteriuria (16). These results suggested that it would be possible to study recurrences of the same strain bacteriuria by using a kinetic analysis of urine bacterial titers. In an initial experiment, five mice from one cage (cage 1) were infected with UTI89, a clinical cystitis strain, via intraurethral inoculation, and urine samples were collected every other day for 6 weeks. Two of the five mice (M1 and M4) had at least one recurrent episode of bacteriuria after urine sterility had been documented (Fig. 1 and Table 1). M2 and M3 had persistent infections with high-titer bacteriuria lasting for 9 and 35 days, respectively. After clearance of the initial bacterial challenge, M5 had no detectable bacteria in the urine throughout the duration of the experiment. All of the culture-positive urine samples contained between 9 × 103 and 3 × 106 CFU/ml, arguing against the possibility of sample contamination by urethral or vaginal organisms. At the end of 6 weeks, the mice were sacrificed, and bacterial titers in the bladders were determined. Interestingly, M1, M4, and M5 all had bacteria persisting in their bladder tissue; however, the bladders of M2 and M3 were sterile (Fig. 2). Moreover, the left kidney of M2 was shrunken and scarred. The kidneys from all of the mice were sterile (data not shown).
FIG. 1.
Kinetic analysis of urine and feces after infection with UPEC. C57BL/6 mice were infected with UTI89 (M1 to M5) or UTI89 SR (M6 to M15), and bacterial titers in urine (▪) and feces (□) samples were determined every other day for 6 weeks (42 days). Five mice were together in each cage with M1 to M5 (cage 1), M6 to M10 (cage 2), and M11 to M15 (cage 3). M10 was not infected with UTI89 SR. ✽, No urine collected; ♦, no feces collected.
TABLE 1.
Recurrent and persistent bacteriuria in mice after infection with UPEC
| Mouse no. | Day(s) after infection of bacteriuric events |
|---|---|
| M1 | 22 |
| M2 | 2-35 |
| M3 | 2-9 |
| M4 | 7, 16 |
| M7 | 11, 15, 25-26, 30 |
| M8 | 1.5-9 |
| M12 | 13, 26 |
| M13 | 13 |
FIG. 2.
Bacterial persistence in the bladders of mice infected with UPEC. Bladders were removed from the indicated mice 6 weeks after intraurethral infection with UTI89 (M1 to M5) or UTI89 SR (M6 to M15), and the bacterial titers in the tissue were determined. K, Kidney scarring; B, persistent bacteriuria; U, uninfected.
To conclusively determine that these recurrences were caused by the inoculated strain and to assess whether a fecal reservoir of UPEC was established in this model of acute UTI, nine mice (cage 2, M6 to M9; cage 3, M11 to M15) were infected with a streptomycin-resistant strain of UTI89 (UTI89 SR). One mouse, M10, was left uninfected but remained in cage 2 throughout the experiment. Importantly, no streptomycin-resistant bacteria could be isolated from the bladder, feces, or urine of uninfected mice or from mice infected with a streptomycin-sensitive UPEC strain (unpublished observations). Urine and fecal samples were collected every other day for 6 weeks, and the results are shown in Fig. 1 and Table 1. Three of the nine infected mice had recurrent episodes of bacteriuria after documented urine sterility with M13 having one episode, M12 having two episodes, and M7 having four episodes. As before, one of the mice, M8, had a persistent infection that did not clear until day 9 after infection. The remainder of the mice did not have any documented recurrences. To further demonstrate that the streptomycin-resistant E. coli were the same as the inoculated strain, arbitrarily primed PCR DNA fingerprinting was performed on random isolates from bladder tissue, urine fluxes, and fecal samples (12). All of the isolates showed the same banding pattern as the inoculated strain (data not shown).
The analysis of fecal samples demonstrated that all of the mice became transiently or continuously colonized with the infecting strain of UPEC, including the uninfected mouse, M10 (Fig. 1). Recurrent bacteriuria was associated with elevations of fecal titers in mice sharing the same cage. At the end of 6 weeks, the mice were sacrificed and bladder bacterial titers were determined as before. All of the mice infected with UTI89 SR (mice 6 to 15) were still colonized, except for M10 and M12, who had sterile bladders (Fig. 2). The right kidney of M12 was distorted and showed evidence of abscesses and scarring, and M10 was an uninfected control. None of the kidneys from these mice were infected at the 6-week time point (data not shown).
Transmission of UPEC from the UT to the GIT.
The fact that infected mice became colonized both in their bladders and GITs makes it difficult to determine the source of bacteria causing the recurrent episodes of bacteriuria. However, to address the efficiency of GIT-to-UT transmission, as well as the mouse-to-mouse spread of UPEC, one mouse of five in a cage was infected intraurethrally with UTI89 SR. Fecal samples collected before the infection demonstrated that SR E. coli was not present in the feces of any of the mice (Table 2). The day after infection, fecal samples were again taken from all of the mice, and the fecal titers were determined. Six of the seven uninfected mice had streptomycin-resistant E. coli in their feces 24 h after UTI89 SR was introduced to their environment (Table 2). At 7 days after infection, fecal titers were again determined and demonstrated that only the mice that were infected intraurethrally, M16 and M21, maintained fecal colonization. At 14 days after infection, M16 to M20 were sacrificed and their bladders, urines, and feces were cultured for UTI89 SR. All of the urines were sterile, and only the infected mouse had bacteria in the bladder tissue or in the feces (data not shown and Table 2).
TABLE 2.
Intra- and intermouse transfer of UPEC from the UT to GITa
| Mouse no. | Day −1 fecal titer (CFU/feces) | Day +1 fecal titer (CFU/feces) | Day +7 fecal titer (CFU/feces) | Day +14
|
|
|---|---|---|---|---|---|
| Fecal titer (CFU/feces) | Bladder titer (CFU/bladder) | ||||
| M16* | 0 | 2,000 | 482 | 207 | 310 |
| M17 | 0 | ND | 0 | 0 | 0 |
| M18 | 0 | 414 | 0 | 0 | 0 |
| M19 | 0 | 488 | 0 | 0 | 0 |
| M20 | 0 | 500 | 0 | 0 | 0 |
| M21* | 0 | 500 | 800 | ||
| M22 | 0 | 0 | 0 | ||
| M23 | 0 | 5 | 0 | ||
| M24 | 0 | 27 | 0 | ||
| M25 | 0 | 63 | ND | ||
*,infected mice. M16 to M20 were in one cage, and M21 to M25 were in another cage. ND, not done.
Since transient fecal colonization may not be sufficient to facilitate GIT-to-UT transfer of UPEC, M22 to M25 were given an anal lavage with UTI89 SR in an attempt to establish fecal persistence of the uropathogen. At days 14, 21, and 25 after inoculation four of four lavaged mice still had UTI89 SR still in their feces (Table 3). Since 90% of the recurrent episodes of bacteriuria occurred by day 25 after infection, these mice were sacrificed at day 25, and the titers of feces, urine, and bladder samples were determined for UTI89 SR. Importantly, it has been demonstrated that instillation of as few as 105 CFU/ml of UTI89 into the bladders of C57BL/6 mice establishes bladder colonization for at least 1 month after the acute infection (data not shown); therefore, if any of the mice with persistent fecal colonization developed an acute UTI during the experiment (where bacterial titers often exceed 107 CFU/ml), bacteria would be expected to remain in the bladder. In this way, it is possible to assess the efficiency of GIT-to-UT transmission in this experimental system. At day 25, only the mouse that had undergone intraurethral infection had UTI89 SR in the bladder (Table 3). All of the urine samples were sterile at this time point (data not shown).
TABLE 3.
GIT colonization with UPEC does not lead to efficient spread to the UTa
| Mouse no. | Fecal titer (CFU) at:
|
Bladder titer (CFU) at day 25 | ||||
|---|---|---|---|---|---|---|
| Day 3 | Day 7 | Day 14 | Day 21 | Day 25 | ||
| M21* | 32 | 312 | 10 | 15 | 0 | 510 |
| M22 | ND | 86 | 64 | 16 | 74 | 0 |
| M23 | 107 | 1,012 | 110 | 845 | 64 | 0 |
| M24 | 1,500 | 1,480 | 1,500 | 446 | 1,120 | 0 |
| M25 | 402 | 197 | 1,100 | 282 | 8 | 0 |
*, intraurethrally infected mouse. ND, not done.
To exclude the possibility that an initial bladder infection renders the UT more amenable to colonization with GIT bacteria, C57BL/6 mice were initially infected with UTI89; at 2 weeks after infection the mice were subsequently given an anal-lavage, periurethral-vaginal, or intraurethral inoculation with NU14, a streptomycin-resistant cystitis isolate. Mice that were previously infected with UTI89 were significantly more resistant to colonization with NU14 instilled intraurethrally compared to mock-infected animals [median values, 5.4 × 102 CFU/bladder (UTI89 preinfected) versus 1.5 × 105 CFU/bladder (mock infected), P ≤ 0.05]. The mice that were given anal lavage or periurethral colonization did not have any streptomycin-resistant bacteria in their bladders at day 28 after instillation of NU14. However, all of the mice were still colonized with UTI89 (data not shown).
The effect of 3- versus 10-day SXT therapy on UPEC persistence and recurrence.
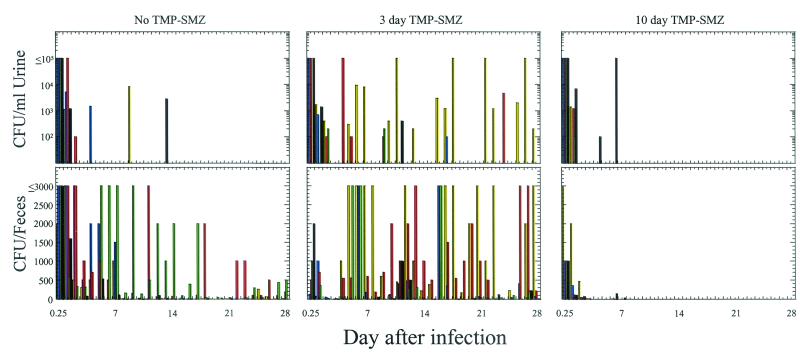
To determine the effects of antibiotic therapy on urine recurrences, as well as persistence of UPEC in the bladder and feces, C57BL/6 mice were infected with UTI89 SR and were subsequently left untreated or treated with SXT in their drinking water for 3 days or 10 days. Urine and fecal samples were collected twice on the first day after infection and on each subsequent day for 28 days. Regardless of the antibiotic therapy, C57BL/6 mice rapidly cleared UTI89 SR from the urine, as had been observed previously (16) (Fig. 3). The five mice that received no antibiotics had three urinary recurrences of >103 CFU/ml in three different mice on days 4 (M27), 9 (M26), and 13 (M30) after infection. Fecal titers were present in the majority of mice in this group throughout the observation period. After the last urinary recurrence, on day 13 postinfection, the fecal titers progressively decreased.
FIG. 3.
Effect of SXT on bacteriuric recurrences and the fecal reservoir. C57BL/6 mice were infected intraurethrally with UTI89 SR and subsequently treated with SXT for 3 days (M31 to M35) or 10 days (M36 to M40), or left untreated (M26 to M30). Urine and fecal samples were collected at 6 h after infection and daily thereafter for 28 days, and then titers were determined for the CFU of UTI89 SR. Mouse key: M26, M31, and M36 (yellow bars); M27, M32, and M37 (blue bars); M28, M33, and M38 (red bars); M29, M34, and M39 (green bars); M30, M35, and M40 (black bars).
In the five mice that received 3 days of SXT, fecal titers were reduced to <10 CFU/feces in all of the mice (mean, 2.5 CFU/feces) by the end of 3 days of antibiotic therapy. On day 4 after infection, within 1 day after the cessation of antibiotic treatment, M33 had a urinary recurrence of UTI89 SR, and on days 5 to 7 M31 also had a recurrent episode of bacteriuria, both of which were >104 CFU/ml of urine (Fig. 3). The episodes of bacteriuria in these mice were associated with an increase in fecal titers for all of the mice within their cage. During the rest of the experiment there were five additional bacteriuric recurrences that were >103 CFU/ml; four were in M31 (days 10 to 11, 16 to 18, 22 to 23, and 26 to 28) and one was in M33 (day 24).
The mice that were treated with SXT for 10 days, like those treated for 3 days, rapidly cleared UTI89 SR from the feces. Interestingly, M40 had two urinary recurrences of UTI89 SR while on antibiotic therapy, on days 4 and 6 after infection, but only one was >103 CFU/ml of urine (Fig. 3). Also on day 6, fecal titers reappeared in three of five mice within the cage; however, the magnitude of fecal colonization was substantially lower than that typically associated with recurrent episodes of bacteriuria in the absence of antibiotics and was rapidly cleared after the bacteriuric episode subsided (Fig. 3). Even after SXT was removed from the water at day 10, there was no culturable UTI89 SR detected in either the urine or the feces throughout the rest of the experiment.
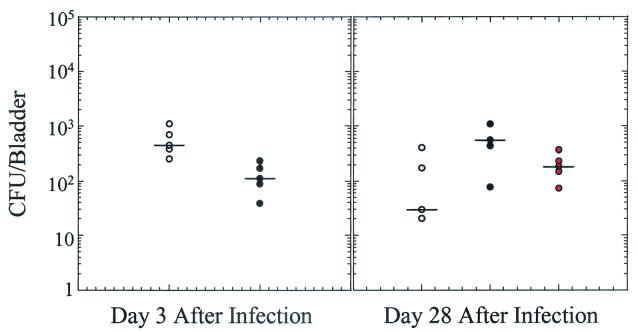
To test the effect of SXT therapy on bacterial persistence in the bladder, mice with different treatment regimens were sacrificed at day 3 after infection and at day 28 after infection and bacterial titers in the bladders were determined. At day 3 after infection the bladder bacterial titers were about four times lower for the SXT-treated group than the untreated group (median values, 420 CFU/bladder [untreated] versus 110 CFU bladder [treated]); however, all of the treated mice were still colonized (Fig. 4). Intriguingly, at day 28 after infection all of the mice were still colonized with UTI89 SR in their bladders irrespective of the duration antibiotic therapy (median values, 30 CFU/ml [untreated], 440 CFU/ml [3 day treated], and 180 CFU/ml [10 day treated]).
FIG. 4.
Effect of SXT on bacterial persistence in the bladder. C57BL/6 mice were infected with UTI89 SR and treated with SXT for 3 days (black circles) or 10 days (red circles) or were left untreated (white circles). Bladders were harvested at days 3 and 28 after infection, and serial dilutions of the bladder homogenates were titered to determine the level of culturable UTI89 SR remaining in the bladder. The black bars indicate the median values.
DISCUSSION
The high propensity for UTIs to recur poses a significant challenge to the clinical management of these infections. The current thought is that all recurrent UTIs result from reinoculation of the UT with bacteria from the GIT or vaginal-periurethral flora. In support of this model is epidemiologic evidence demonstrating that UPEC strains can be found in the GIT and the vaginal-periurethral areas before and/or after an acute UTI (18, 25). Furthermore, women who suffer from recurrent UTIs tend to have a higher level of vaginal colonization with E. coli than do women who do not have recurrent UTIs (22). However, it has been difficult to prove that recurrent infections are solely the result of reinfections from an exogenous site by using epidemiologic approaches, especially given the high frequency of same-strain recurrences (1, 4, 10, 11, 18). A recent study demonstrated that a clinical E. coli pyelonephritis isolate could cause chronic infection in the lipopolysaccharide-hyporesponsive C3H/HeJ mouse strain (5). The data presented in the present study of recurrent bacteriuria in wild-type mice suggest that recurrent infections with the same strain of E. coli may be a manifestation of chronic bladder colonization.
After intraurethral inoculation of C57BL/6 mice with UTI89, a clinical cystitis isolate, the bacteria are rapidly cleared from the urine. However, we observed that after the initial clearance of bacteria from the urine, recurrent bacteriuria occurred in 36% (5 of 14) of the mice, and multiple bacteriuric episodes occurred in 22% (3 of 13) of the mice. The majority of the bacteriuric episodes had urine titers of <104 CFU of E. coli/ml. By using a streptomycin-resistant strain of UTI89 it was determined that all of the mice, including an uninfected mouse in the same cage as the infected mice, developed either transient or continuous fecal colonization with this E. coli strain after intraurethral inoculation. Arbitrarily primed PCR DNA fingerprinting was also used to further show that the E. coli isolates from the urine and feces were identical to the inoculated strain. Thus, in this animal model, bladder and fecal UPEC reservoirs are established after acute infection with UTI89. The most likely mode of UPEC transmission from the UT to the GIT is via a urine-oral route; however, it is possible that alternate routes exist for bacterial spread from the UT to the GIT. The vaginal reservoir of UPEC was not directly assessed in the present study and will be the focus of future investigations.
The transmission of UTI89 SR from infected mice to the GIT of uninfected mice is an efficient process, as 86% (six of seven) of the uninfected mice produced fecal specimens that became positive for UTI89 SR after exposure to an acutely infected cagemate. However, 1 week later the same uninfected mice no longer had UTI89 SR in their feces. However, the feces of both of the infected mice remained colonized, suggesting that maintenance of the fecal titer is enhanced by the persistence of bacteria in the bladder and that UPEC in the feces at levels of 102 CFU/feces is unable to efficiently colonize the GIT of other animals. When the mice (M16 to M20) were sacrificed at day 14 after the introduction of the infected mouse, only the infected mouse (M16) had UTI89 SR in the bladder, suggesting that GIT-to-UT transmission had not occurred in M17 to M20. M16 still had significant numbers of UTI89 SR in its feces but at decreased levels compared to the initial colonization level (Table 2).
Because of the intimate relationship between fecal colonization and bacteriuria, it is difficult to conclusively state which event facilitates the other. Regardless, these observations suggest that transmission from the UT to the GIT may be a means by which UPEC maintains itself within a particular host and illustrates the complex host-pathogen network established during an acute infection with UPEC. In addition, the fact that M10 maintained bladder sterility despite having fecal colonization with UTI89 and being in a cage with four other infected mice for 6 weeks suggests that spread from the feces to the UT is a rare event in this experimental system. Further support for this conclusion comes from the anal lavage experiments, wherein fecal colonization was established in the absence of bladder colonization, and the UTs of these mice remained sterile up to 25 days postlavage. In fact, these data are consistent with results from epidemiologic studies in which it has been shown that the detection of UPEC isolates in the feces does not predict the risk of a UTI (25).
Acute UTIs are treated with antibiotics, such as SXT (7). The majority of the time antibiotics clear bacteriuria and result in the resolution of symptoms. However, despite appropriate antibiotic therapy UTIs still recur. To test the impact of SXT therapy on the bladder and fecal reservoirs after an acute UTI, mice were treated with SXT for 3 days or 10 days or left untreated after intraurethral inoculation with UTI89 SR. Mice who did not receive antibiotics or received a 3-day course of antibiotics had several urinary recurrences of <103 CFU/ml. In contrast, of the five mice that received a 10-day course of SXT there was only one urinary recurrence of <103 CFU/ml, which occurred during antibiotic therapy. SXT therapy was associated with a rapid decline in the fecal titers of UTI89 SR; however, the mice that received only 3 days of therapy had a resurgence of fecal colonization that was associated with UTI89 SR reappearing in the urine. In contrast, the mice that received 10 days of antibiotic therapy had only one mild and short-lived fecal reemergence; however, it was also associated with the episode of recurrent bacteriuria in this treatment group. Interestingly, there were no urinary recurrences or detectable fecal titers in the 10-day-SXT-treated group between days 10 and 28 after infection, after the antibiotic had been removed. Together, these data demonstrate that 3 days of SXT treatment does not alter the course of urinary recurrences in this mouse model, despite clearing UPEC from the fecal reservoir and reducing bacterial titers in the bladder during the acute phase of the infection. However, 10 days of SXT therapy appeared to reduce urinary recurrences of UTI89 SR and eradicated the fecal reservoir through day 28 after infection. Importantly, all of the mice were still colonized with UTI89 SR in the bladder at day 28 after infection irrespective of antibiotic therapy, demonstrating that the bacterial reservoir is very stable even in the face of antibiotics. The persistence of UTI89 SR after 10 days of antibiotic therapy raises the possibility that at some later time these bacteria could serve as a seed for a subsequent recurrent bacteriuric episode. When interpreting these results with respect to the recurrent UTIs in a clinical population, it is important to remember that the environment in a mouse cage is quite distinct from most human habitations, necessitating future analysis testing the clinical relevance of these results. Irrespective of this fact, these findings illustrate that the interplay between the GIT and the UT is complex and clearly show that the persistence of bacteria in the bladder significantly alters the risk of recurrent bacteriuria and persistence of bacteria in the GIT. These findings are particularly interesting in light of the recent discovery that a particular uropathogenic clone of E. coli has disseminated to several locations within the United States (13). In addition, 10-day SXT therapy is more effective at preventing bacteriuric recurrences and subsequent reseeding of the GIT than 3 days of SXT therapy; however, neither of these regimens eradicates UTI89 SR from the bladder reservoir. Future studies designed at investigating the bacteriologic properties of persistent E. coli, as well as the potential triggers for bacterial reemergence from the bladder reservoir, could facilitate the development of new treatment modalities for recurrent UTIs focused on the eradication of persistent bacteria from the bladder.
Acknowledgments
This work was supported by NIH grants RO1DK51406 (S.J.H.), RO1A129549 (S.J.H.), RO1AI48689 (S.J.H.), R21DK57926 (R.G.L.), and R21DK57936 (R.G.L.).
We thank Matt Mulvey, Matt Chapman, and Steven Martin for many helpful discussions and critical review of the manuscript.
Editor: A. D. O'Brien
REFERENCES
- 1.Brauner, A., S. H. Jacobson, and I. Kuhn. 1992. Urinary Escherichia coli causing recurrent infections: a prospective follow-up of biochemical phenotypes. Clin. Nephrol. 38:318-323. [PubMed] [Google Scholar]
- 2.Cass, A. S., and G. W. Ireland. 1985. Antibacterial perineal washing for prevention of recurrent urinary tract infections. Urology XXV:492-494. [DOI] [PubMed]
- 3.Foxman, B. 1990. Recurring urinary tract infection: incidence and risk factors. Am. J. Public Health 80:331-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foxman, B., B. Gillespie, J. Koopman, L. Zhang, K. Palin, P. Tallman, J. V. Marsh, S. Spear, J. D. Sobel, M. J. Marty, and C. F. Marrs. 2000. Risk factors for second urinary tract infection among college women. Am. J. Epidemiol. 151:1194-1205. [DOI] [PubMed] [Google Scholar]
- 5.Goluszko, P., S. L. Moseley, L. D. Truong, A. Kaul, J. R. Williford, R. Selvarangan, S. Nowicki, and B. Nowicki. 1997. Development of experimental model of chronic pyelonephritis with Escherichia coli O75:K5:H-bearing Dr fimbriae: mutation in the dra region prevented tubulointerstitial nephritis. J. Clin. Investig. 99:1662-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hooton, T. M. 2001. Recurrent urinary tract infection in women. Int. J. Antimicrob. Agents 17:259-268. [DOI] [PubMed] [Google Scholar]
- 7.Hooton, T. M., and W. E. Stamm. 1997. Diagnosis and treatment of uncomplicated urinary tract infection. Infect. Dis. Clin. N. Am. 11:551-581. [DOI] [PubMed] [Google Scholar]
- 8.Hultgren, S. J., T. N. Porter, A. J. Schaeffer, and J. L. Duncan. 1985. Role of type 1 pili and effects of phase variation on lower urinary tract infections produced by Escherichia coli. Infect. Immun. 50:370-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hung, C. S., J. Bouckaert, D. Hung, J. Pinkner, C. Widberg, A. DeFusco, C. G. Auguste, R. Strouse, S. Langermann, G. Waksman, and S. J. Hultgren. 2002. Structural basis of tropism of Escherichia coli to the bladder during urinary tract infection. Mol. Microbiol. 44:903-915. [DOI] [PubMed] [Google Scholar]
- 10.Ikaheimo, R., A. Siitonen, T. Heiskanen, U. Karkkainen, P. Kuosmanen, P. Lipponen, and P. H. Makela. 1996. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin. Infect. Dis. 22:91-99. [DOI] [PubMed] [Google Scholar]
- 11.Karkkainen, U. M., R. Ikaheimo, M. L. Katila, and A. Siitonen. 2000. Recurrence of urinary tract infections in adult patients with community-acquired pyelonephritis caused by E. coli: a 1-year follow-up. Scand. J. Infect. Dis. 32:495-499. [DOI] [PubMed] [Google Scholar]
- 12.Madico, G., N. S. Akopyants, and D. E. Berg. 1995. Arbitrarily primed PCR DNA fingerprinting of Escherichia coli O157:H7 strains by using templates from boiled cultures. J. Clin. Microbiol. 33:1534-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manges, A. R., J. R. Johnson, B. Foxman, T. T. O'Bryan, K. E. Fullerton, and L. W. Riley. 2001. Widespread distribution of urinary tract infections caused by a multidrug-resistant Escherichia coli clonal group. N. Engl. J. Med. 345:1007-1013. [DOI] [PubMed] [Google Scholar]
- 14.McCarty, J. M., G. Richard, W. Huck, R. M. Tucker, R. L. Tosiello, M. Shan, A. Heyd, and R. M. Echols. 1999. A randomized trial of short-course ciprofloxacin, ofloxacin, or trimethoprim-sulfamethoxazole for the treatment of acute urinary tract infection in women. Am. J. Med. 106:292-299. [DOI] [PubMed] [Google Scholar]
- 15.Mulvey, M. A., Y. S. Lopez-Boado, C. L. Wilson, R. Roth, W. C. Parks, J. Heuser, and S. J. Hultgren. 1998. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282:1494-1497. [DOI] [PubMed] [Google Scholar]
- 16.Mulvey, M. A., J. D. Schilling, and S. J. Hultgren. 2001. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect. Immun. 69:4572-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulvey, M. A., J. D. Schilling, J. J. Martinez, and S. J. Hultgren. 2000. From the cover: bad bugs and beleaguered bladders: interplay between uropathogenic Escherichia coli and innate host defenses. Proc. Natl. Acad. Sci. USA 97:8829-8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russo, T. A., A. Stapleton, S. Wenderoth, T. M. Hooton, and W. E. Stamm. 1995. Chromosomal restriction fragment length polymorphism analysis of Escherichia coli strains causing recurrent urinary tract infections in young women. J. Infect. Dis. 172:440-445. [DOI] [PubMed] [Google Scholar]
- 19.Sauer, F. G., M. A. Mulvey, J. D. Schilling, J. J. Martinez, and S. J. Hultgren. 2000. Bacterial pili: molecular mechanisms of pathogenesis. Curr. Opin. Microbiol. 3:65-72. [DOI] [PubMed] [Google Scholar]
- 20.Schaeffer, A. J., J. M. Jones, and J. K. Dunn. 1981. Association of vitro Escherichia coli adherence to vaginal and buccal epithelial cells with susceptibility of women to recurrent urinary-tract infections. N. Engl. J. Med. 304:1062-1066. [DOI] [PubMed] [Google Scholar]
- 21.Schlager, T. A., J. O. Hendley, J. A. Lohr, and T. S. Whittam. 1993. Effect of periurethral colonization on the risk of urinary tract infection in healthy girls after their first urinary tract infection. Pediatr. Infect. Dis. J. 12:988-993. [DOI] [PubMed] [Google Scholar]
- 22.Stamey, T. A., and C. C. Sexton. 1975. The role of vaginal colonization with enterobacteriaceae in recurrent urinary infections. J. Urol. 113:214-217. [DOI] [PubMed] [Google Scholar]
- 23.Stamm, W. E., T. M. Hooton, J. R. Johnson, C. Johnson, A. Stapleton, P. L. Roberts, S. L. Moseley, and S. D. Fihn. 1989. Urinary tract infections: from pathogenesis to treatment. J. Infect. Dis. 159:400-406. [DOI] [PubMed] [Google Scholar]
- 24.Stamm, W. E., M. McKevitt, P. L. Roberts, and N. J. White. 1991. Natural history of recurrent urinary tract infections in women. Rev. Infect. Dis. 13:77-84. [DOI] [PubMed] [Google Scholar]
- 25.Stapleton, A., T. M. Hooton, C. Fennell, P. L. Roberts, and W. E. Stamm. 1995. Effect of secretor status on vaginal and rectal colonization with fimbriated Escherichia coli in women with or without recurrent urinary tract infection. J. Infect. Dis. 171:717-720. [DOI] [PubMed] [Google Scholar]