Abstract
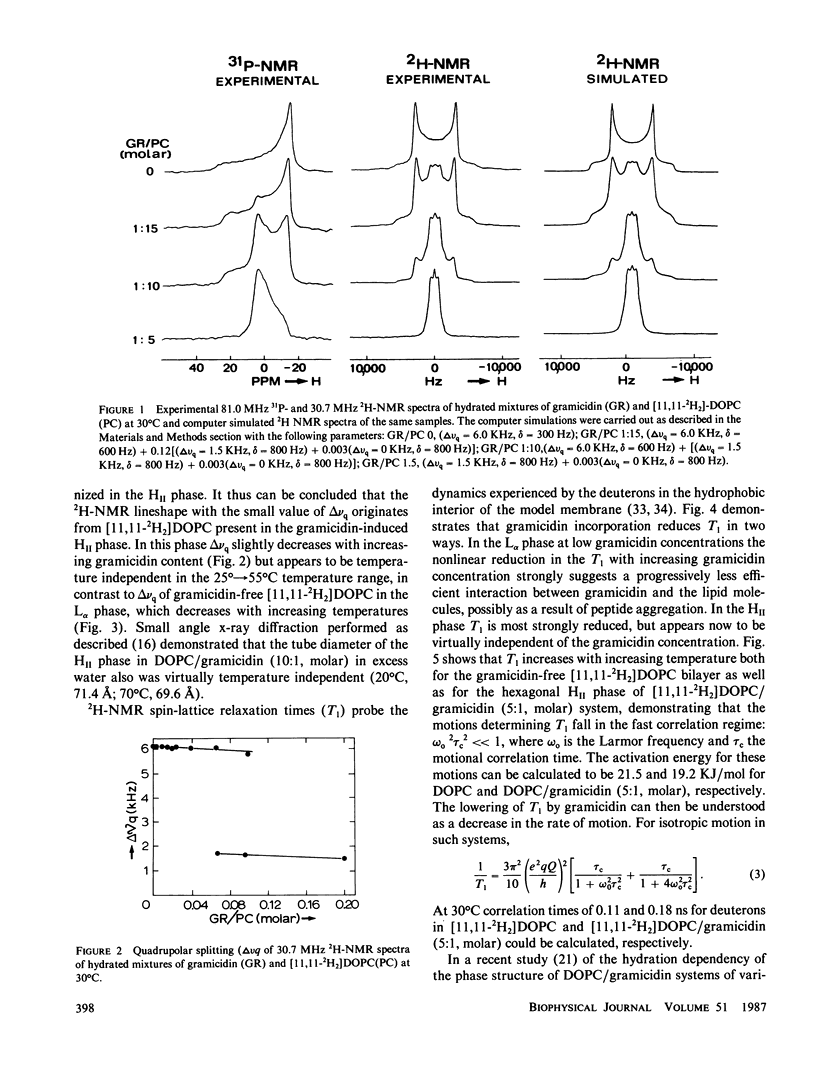
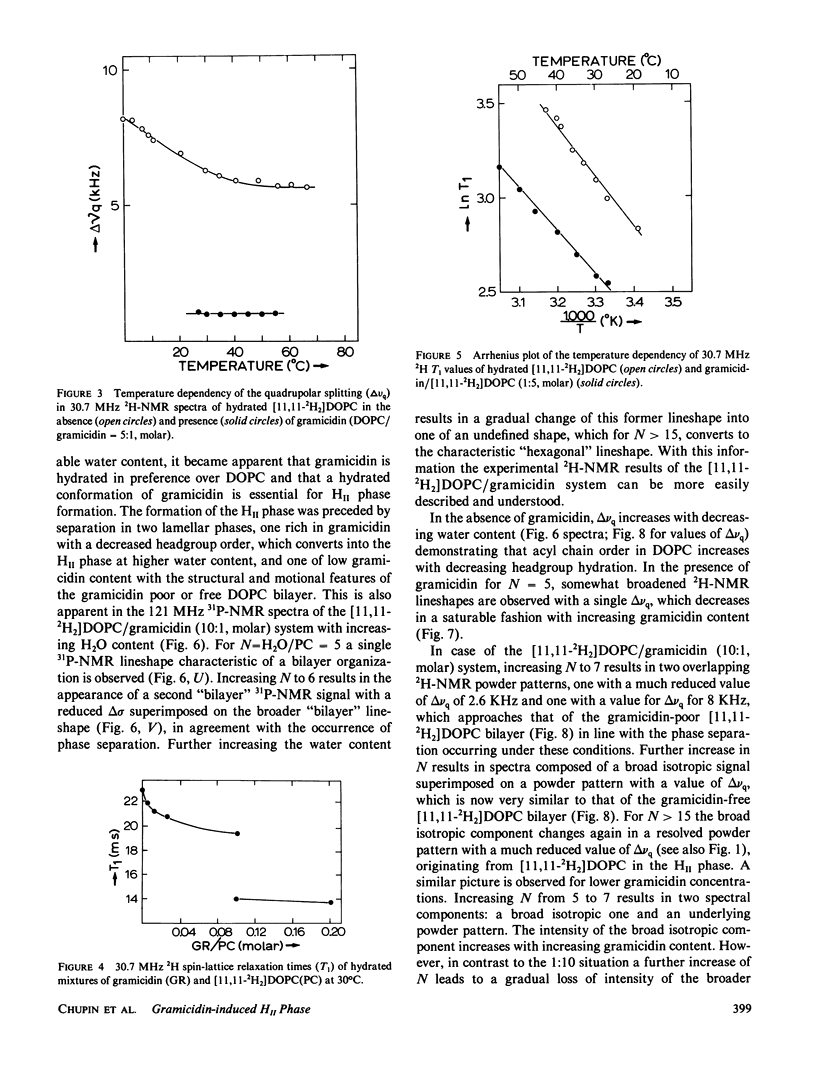
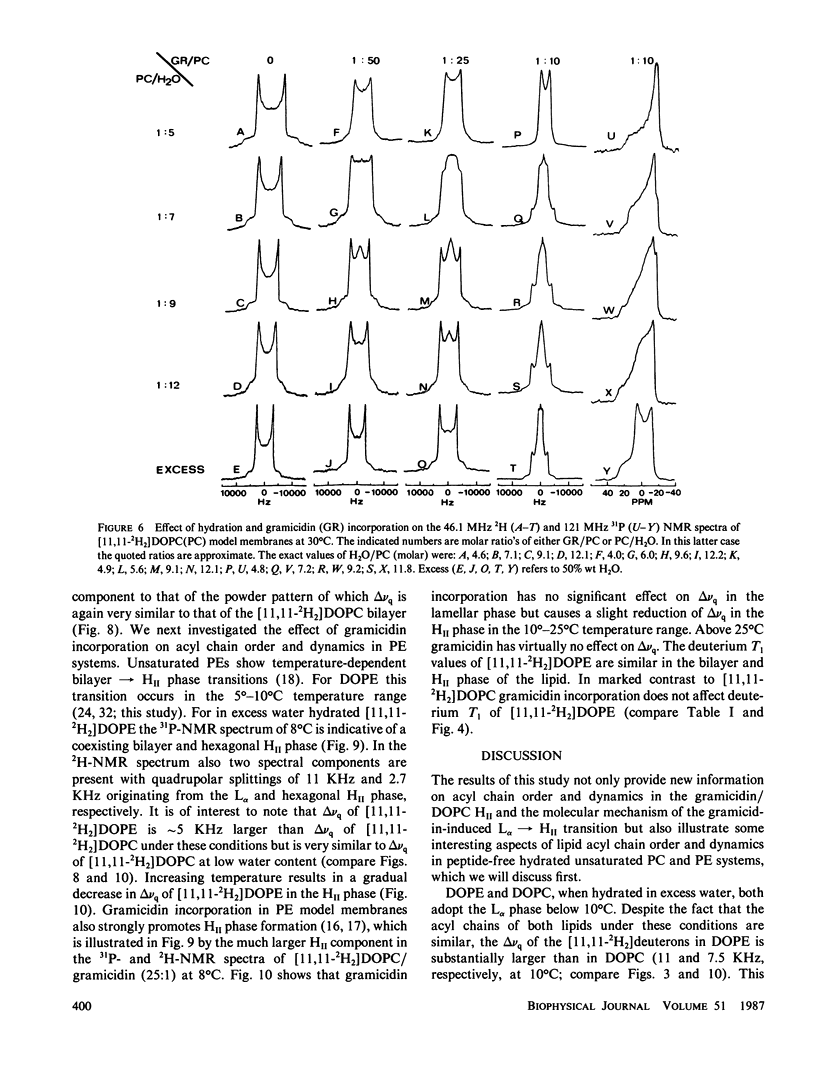
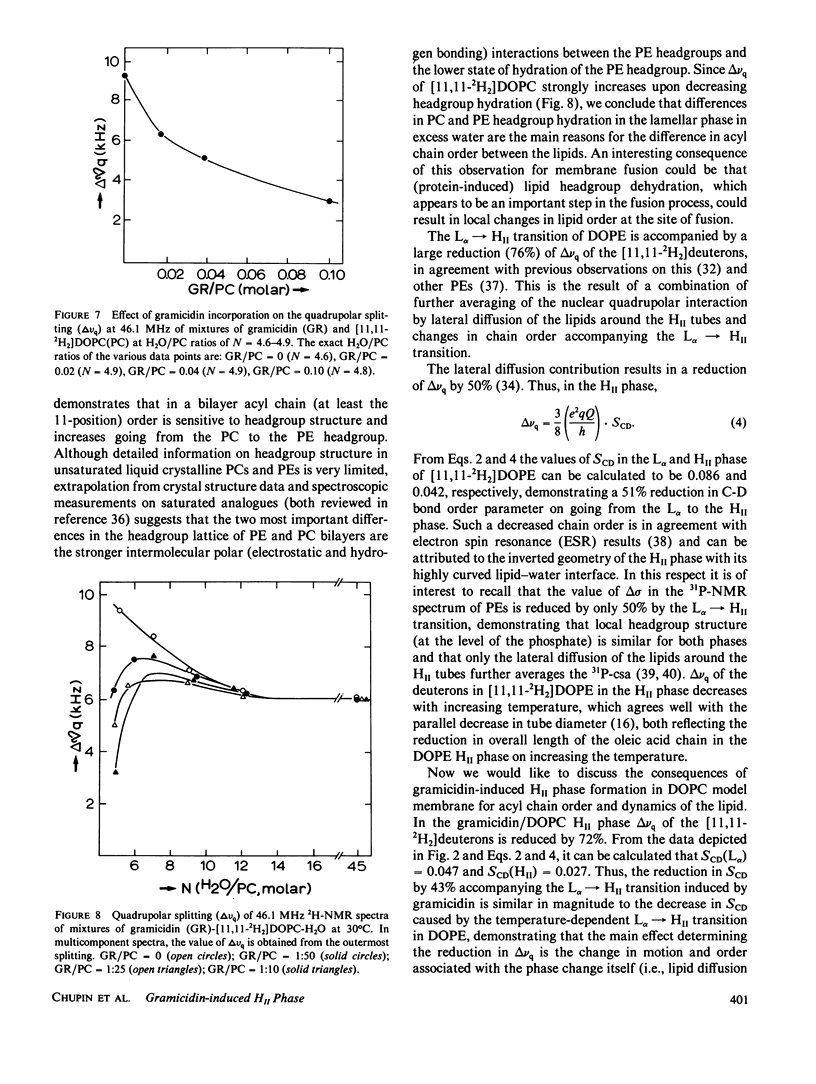
The following results are reported in this paper: The interaction of gramicidin with [11,11-2H2]dioleoylphosphatidylcholine (DOPC) and [11,11-2H2]dioleoylphosphatidylethanolamine (DOPE) at different stages of hydration was studied by 2H- and 31P-nuclear magnetic resonance. In the L alpha phase in excess water the acyl chains of phosphatidylethanolamine (PE) are more ordered than phosphatidylcholine (PC) most likely as the result of the lower headgroup hydration of the former lipid. In excess water gramicidin incorporation above 5 mol % in DOPC causes a bilayer----hexagonal HII phase change. In the HII phase acyl chain order is virtually unaffected by gramicidin but the peptide restricts the fast chain motions. At low water content gramicidin cannot induce the HII phase but it markedly decreases chain order in the DOPC bilayer. Increasing water content results in separation between a gramicidin-poor and a gramicidin-rich L alpha phase with decreased order of the entire lipid molecule. Further increase in hydration reverts at low gramicidin contents the phase separation and at high gramicidin contents results in a direct change of the disordered lamellar to the hexagonal HII phase. Gramicidin also promotes HII phase formation in the PE system but interacts much less strongly with PE than with PC. The results support our hypothesis that gramicidin, by a combination of strong intermolecular attraction forces and its pronounced cone shape, both involving the four tryptophans at the COOH-terminus, has a strong tendency to organize, with the appropriate lipid, in intramembranous cylindrical structures such as is found in the HII phase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen O. S. Gramicidin channels. Annu Rev Physiol. 1984;46:531–548. doi: 10.1146/annurev.ph.46.030184.002531. [DOI] [PubMed] [Google Scholar]
- Chapman D., Cornell B. A., Ellasz A. W., Perry A. Interactions of helical polypepetide segments which span the hydrocarbon region of lipid bilayers. Studies of the gramicidin A lipid-water system. J Mol Biol. 1977 Jul 5;113(3):517–538. doi: 10.1016/0022-2836(77)90236-4. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., De Kruyff B. 31P NMR studies of unsonicated aqueous dispersions of neutral and acidic phospholipids. Effects of phase transitions, p2H and divalent cations on the motion in the phosphate region of the polar headgroup. Biochim Biophys Acta. 1976 Jul 1;436(3):523–540. doi: 10.1016/0005-2736(76)90438-7. [DOI] [PubMed] [Google Scholar]
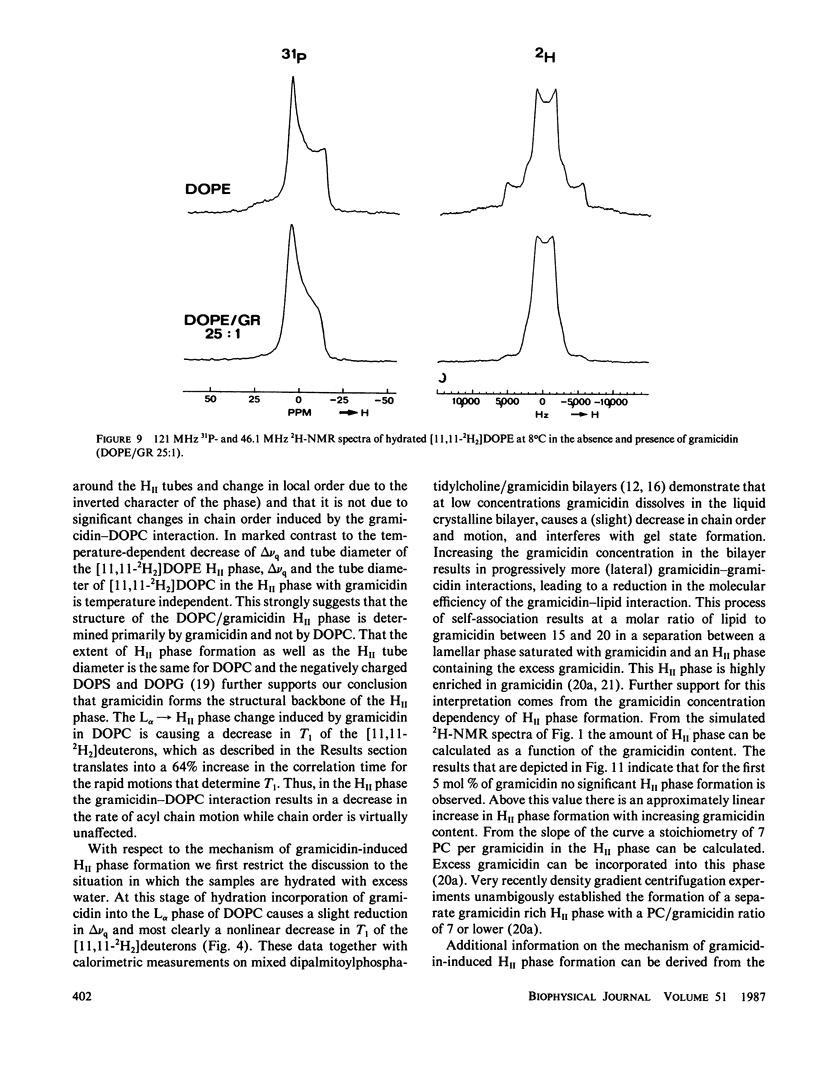
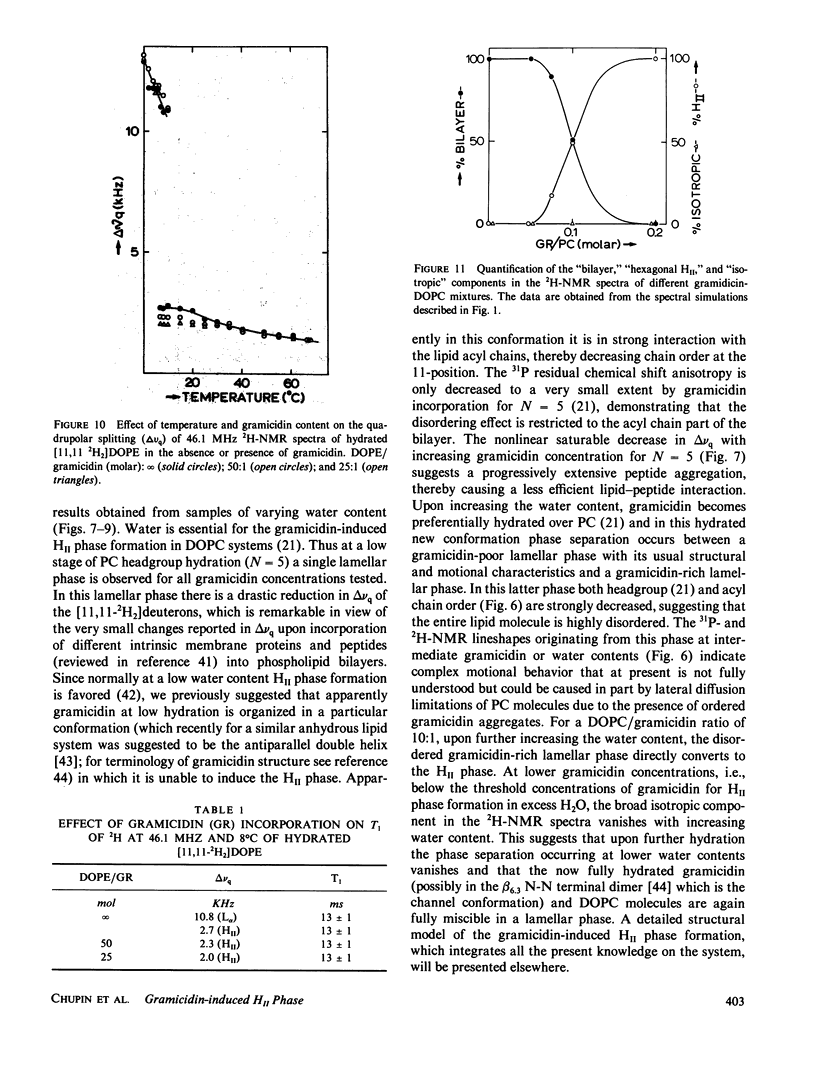
- Cullis P. R., de Kruijff B. Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim Biophys Acta. 1979 Dec 20;559(4):399–420. doi: 10.1016/0304-4157(79)90012-1. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., de Kruijff B. The polymorphic phase behaviour of phosphatidylethanolamines of natural and synthetic origin. A 31P NMR study. Biochim Biophys Acta. 1978 Oct 19;513(1):31–42. doi: 10.1016/0005-2736(78)90109-8. [DOI] [PubMed] [Google Scholar]
- Davis J. H. The description of membrane lipid conformation, order and dynamics by 2H-NMR. Biochim Biophys Acta. 1983 Mar 21;737(1):117–171. doi: 10.1016/0304-4157(83)90015-1. [DOI] [PubMed] [Google Scholar]
- Dekker C. J., Geurts van Kessel W. S., Klomp J. P., Pieters J., De Kruijff B. Synthesis and polymorphic phase behaviour of polyunsaturated phosphatidylcholines and phosphatidylethanolamines. Chem Phys Lipids. 1983 Jul;33(1):93–106. doi: 10.1016/0009-3084(83)90012-9. [DOI] [PubMed] [Google Scholar]
- Devaux P. F., Seigneuret M. Specificity of lipid-protein interactions as determined by spectroscopic techniques. Biochim Biophys Acta. 1985 Jun 12;822(1):63–125. doi: 10.1016/0304-4157(85)90004-8. [DOI] [PubMed] [Google Scholar]
- Farren S. B., Hope M. J., Cullis P. R. Polymorphic phase preferences of phosphatidic acid: A 31P and 2H NMR study. Biochem Biophys Res Commun. 1983 Mar 16;111(2):675–682. doi: 10.1016/0006-291x(83)90359-5. [DOI] [PubMed] [Google Scholar]
- Gally H. U., Pluschke G., Overath P., Seelig J. Structure of Escherichia coli membranes. Fatty acyl chain order parameters of inner and outer membranes and derived liposomes. Biochemistry. 1980 Apr 15;19(8):1638–1643. doi: 10.1021/bi00549a018. [DOI] [PubMed] [Google Scholar]
- Hardman P. D. Spin-label characterisation of the lamellar-to-hexagonal (HII) phase transition in egg phosphatidylethanolamine aqueous dispersions. Eur J Biochem. 1982 May;124(1):95–101. doi: 10.1111/j.1432-1033.1982.tb05910.x. [DOI] [PubMed] [Google Scholar]
- Hauser H., Pascher I., Pearson R. H., Sundell S. Preferred conformation and molecular packing of phosphatidylethanolamine and phosphatidylcholine. Biochim Biophys Acta. 1981 Jun 16;650(1):21–51. doi: 10.1016/0304-4157(81)90007-1. [DOI] [PubMed] [Google Scholar]
- Hladky S. B., Haydon D. A. Discreteness of conductance change in bimolecular lipid membranes in the presence of certain antibiotics. Nature. 1970 Jan 31;225(5231):451–453. doi: 10.1038/225451a0. [DOI] [PubMed] [Google Scholar]
- Katz E., Demain A. L. The peptide antibiotics of Bacillus: chemistry, biogenesis, and possible functions. Bacteriol Rev. 1977 Jun;41(2):449–474. doi: 10.1128/br.41.2.449-474.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killian J. A., Borle F., de Kruijff B., Seelig J. Comparative 2H- and 31P-NMR study on the properties of palmitoyllysophosphatidylcholine in bilayers with gramicidin, cholesterol and dipalmitoylphosphatidylcholine. Biochim Biophys Acta. 1986 Jan 16;854(1):133–142. doi: 10.1016/0005-2736(86)90073-8. [DOI] [PubMed] [Google Scholar]
- Killian J. A., Timmermans J. W., Keur S., de Kruijff B. The tryptophans of gramicidin are essential for the lipid structure modulating effect of the peptide. Biochim Biophys Acta. 1985 Oct 24;820(1):154–156. doi: 10.1016/0005-2736(85)90227-5. [DOI] [PubMed] [Google Scholar]
- Killian J. A., de Kruijff B. Importance of hydration for gramicidin-induced hexagonal HII phase formation in dioleoylphosphatidylcholine model membranes. Biochemistry. 1985 Dec 31;24(27):7890–7898. doi: 10.1021/bi00348a007. [DOI] [PubMed] [Google Scholar]
- Killian J. A., de Kruijff B. The influence of proteins and peptides on the phase properties of lipids. Chem Phys Lipids. 1986 Jun-Jul;40(2-4):259–284. doi: 10.1016/0009-3084(86)90073-3. [DOI] [PubMed] [Google Scholar]
- Killian J. A., de Kruijff B. Thermodynamic, motional, and structural aspects of gramicidin-induced hexagonal HII phase formation in phosphatidylethanolamine. Biochemistry. 1985 Dec 31;24(27):7881–7890. doi: 10.1021/bi00348a006. [DOI] [PubMed] [Google Scholar]
- Killian J. A., de Kruijff B., van Echteld C. J., Verkleij A. J., Leunissen-Bijvelt J., de Gier J. Mixtures of gramicidin and lysophosphatidylcholine form lamellar structures. Biochim Biophys Acta. 1983 Feb 9;728(1):141–144. doi: 10.1016/0005-2736(83)90446-7. [DOI] [PubMed] [Google Scholar]
- Killian J. A., van den Berg C. W., Tournois H., Keur S., Slotboom A. J., van Scharrenburg G. J., de Kruijff B. Gramicidin-induced hexagonal HII phase formation in negatively charged phospholipids and the effect of N- and C-terminal modification of gramicidin on its interaction with zwitterionic phospholipids. Biochim Biophys Acta. 1986 May 9;857(1):13–27. doi: 10.1016/0005-2736(86)90094-5. [DOI] [PubMed] [Google Scholar]
- Lee D. C., Durrani A. A., Chapman D. A difference infrared spectroscopic study of gramicidin A, alamethicin and bacteriorhodopsin in perdeuterated dimyristoylphosphatidylcholine. Biochim Biophys Acta. 1984 Jan 11;769(1):49–56. doi: 10.1016/0005-2736(84)90008-7. [DOI] [PubMed] [Google Scholar]
- Naik V. M., Krimm S. Vibrational analysis of the structure of gramicidin A. II. Vibrational spectra. Biophys J. 1986 Jun;49(6):1147–1154. doi: 10.1016/S0006-3495(86)83743-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perly B., Smith I. C., Jarrell H. C. Effects of replacement of a double bond by a cyclopropane ring in phosphatidylethanolamines: a 2H NMR study of phase transitions and molecular organization. Biochemistry. 1985 Feb 12;24(4):1055–1063. doi: 10.1021/bi00325a038. [DOI] [PubMed] [Google Scholar]
- Rice D. M., Hsung J. C., King T. E., Oldfield E. Protein-lipid interactions. High-field deuterium and phosphorus nuclear magnetic resonance spectroscopic investigation of the cytochrome oxidase-phospholipid interaction and the effects of cholate. Biochemistry. 1979 Dec 25;18(26):5885–5892. doi: 10.1021/bi00593a020. [DOI] [PubMed] [Google Scholar]
- Rice D., Oldfield E. Deuterium nuclear magnetic resonance studies of the interaction between dimyristoylphosphatidylcholine and gramicidin A'. Biochemistry. 1979 Jul 24;18(15):3272–3279. doi: 10.1021/bi00582a012. [DOI] [PubMed] [Google Scholar]
- Sarkar N., Paulus H. Function of peptide antibiotics in sporulation. Nat New Biol. 1972 Oct 25;239(95):228–230. doi: 10.1038/newbio239228a0. [DOI] [PubMed] [Google Scholar]
- Seddon J. M., Cevc G., Kaye R. D., Marsh D. X-ray diffraction study of the polymorphism of hydrated diacyl- and dialkylphosphatidylethanolamines. Biochemistry. 1984 Jun 5;23(12):2634–2644. doi: 10.1021/bi00307a015. [DOI] [PubMed] [Google Scholar]
- Seelig J. 31P nuclear magnetic resonance and the head group structure of phospholipids in membranes. Biochim Biophys Acta. 1978 Jul 31;515(2):105–140. doi: 10.1016/0304-4157(78)90001-1. [DOI] [PubMed] [Google Scholar]
- Seelig J. Deuterium magnetic resonance: theory and application to lipid membranes. Q Rev Biophys. 1977 Aug;10(3):353–418. doi: 10.1017/s0033583500002948. [DOI] [PubMed] [Google Scholar]
- Smith R. L., Oldfield E. Dynamic structure of membranes by deuterium NMR. Science. 1984 Jul 20;225(4659):280–288. doi: 10.1126/science.6740310. [DOI] [PubMed] [Google Scholar]
- Spisni A., Pasquali-Ronchetti I., Casali E., Lindner L., Cavatorta P., Masotti L., Urry D. W. Supramolecular organization of lysophosphatidylcholine-packaged Gramicidin A. Biochim Biophys Acta. 1983 Jul 13;732(1):58–68. doi: 10.1016/0005-2736(83)90186-4. [DOI] [PubMed] [Google Scholar]
- Stark G., Strässle M., Takácz Z. Temperature-jump and voltage-jump experiments at planar lipid membranes support an aggregational (micellar) model of the gramicidin A ion channel. J Membr Biol. 1986;89(1):23–37. doi: 10.1007/BF01870893. [DOI] [PubMed] [Google Scholar]
- Susi H., Sampugna J., Hampson J. W., Ard J. S. Laser-Raman investigation of phospholipid-polypeptide interactions in model membranes. Biochemistry. 1979 Jan 23;18(2):297–301. doi: 10.1021/bi00569a010. [DOI] [PubMed] [Google Scholar]
- Tilcock C. P., Bally M. B., Farren S. B., Cullis P. R. Influence of cholesterol on the structural preferences of dioleoylphosphatidylethanolamine-dioleoylphosphatidylcholine systems: a phosphorus-31 and deuterium nuclear magnetic resonance study. Biochemistry. 1982 Sep 14;21(19):4596–4601. doi: 10.1021/bi00262a013. [DOI] [PubMed] [Google Scholar]
- Urry D. W. The gramicidin A transmembrane channel: a proposed pi(L,D) helix. Proc Natl Acad Sci U S A. 1971 Mar;68(3):672–676. doi: 10.1073/pnas.68.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner T. G., Benson A. A. An improved method for the preparation of unsaturated phosphatidylcholines: acylation of sn-glycero-3-phosphorylcholine in the presence of sodium methylsulfinylmethide. J Lipid Res. 1977 Jul;18(4):548–552. [PubMed] [Google Scholar]
- Weinstein S., Wallace B. A., Blout E. R., Morrow J. S., Veatch W. Conformation of gramicidin A channel in phospholipid vesicles: a 13C and 19F nuclear magnetic resonance study. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4230–4234. doi: 10.1073/pnas.76.9.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]



