Abstract
The physical characteristics which govern the water relations of the giant-celled sporangiophore of Phycomyces blakesleeanus were measured with the pressure probe technique and with nanoliter osmometry. These properties are important because they govern water uptake associated with cell growth and because they may influence expansion of the sporangiophore wall. Turgor pressure ranged from 1.1 to 6.6 bars (mean = 4.1 bars), and was the same for stage I and stage IV sporangiophores. Sporangiophore osmotic pressure averaged 11.5 bars. From the difference between cell osmotic pressure and turgor pressure, the average water potential of the sporangiophore was calculated to be about -7.4 bars. When sporangiophores were submerged under water, turgor remained nearly constant. We propose that the low cell turgor pressure is due to solutes in the cell wall solution, i.e., between the cuticle and the plasma membrane. Membrane hydraulic conductivity averaged 4.6 x 10(-6) cm s-1 bar-1, and was significantly greater in stage I sporangiophores than in stage IV sporangiophores. Contrary to previous reports, the sporangiophore is separated from the supporting mycelium by septa which prevent bulk volume flow between the two regions. The presence of a wall compartment between the cuticle and the plasma membrane results in anomalous osmosis during pressure clamp measurements. This behavior arises because of changes in solute concentration as water moves into or out of the wall compartment surrounding the sporangiophore. Theoretical analysis shows how the equations governing transient water flow are altered by the characteristics of the cell wall compartment.
Full text
PDF

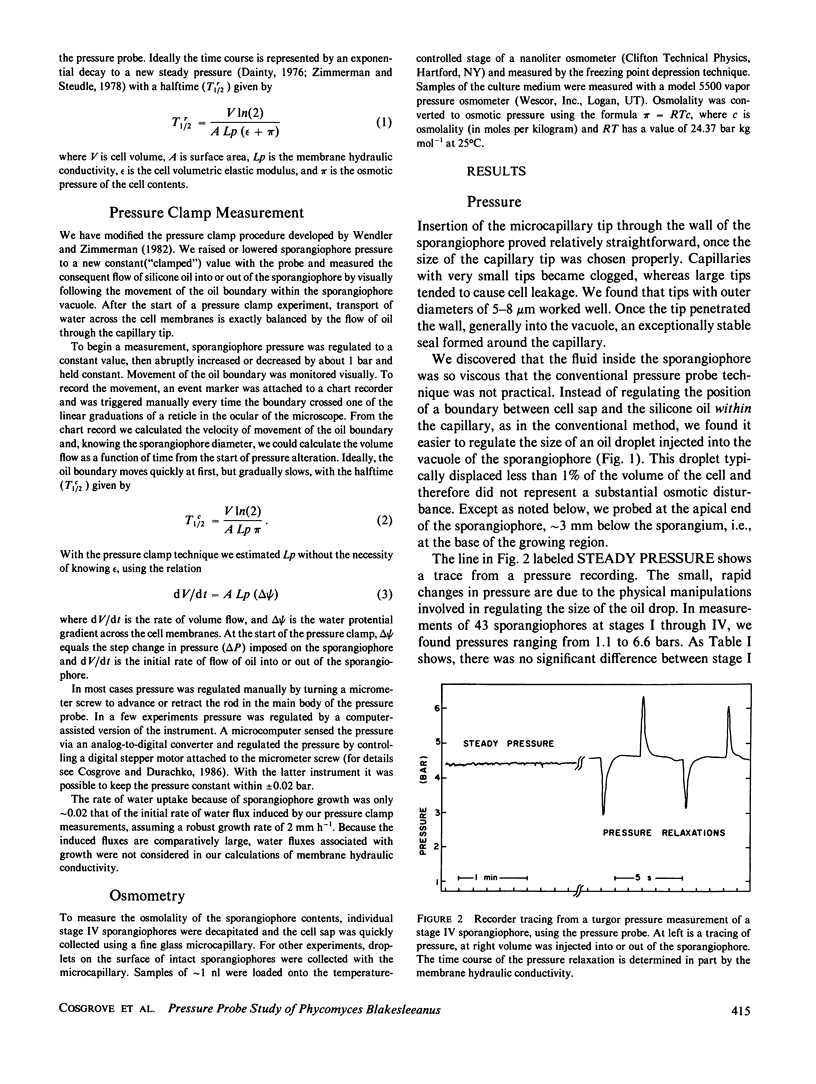
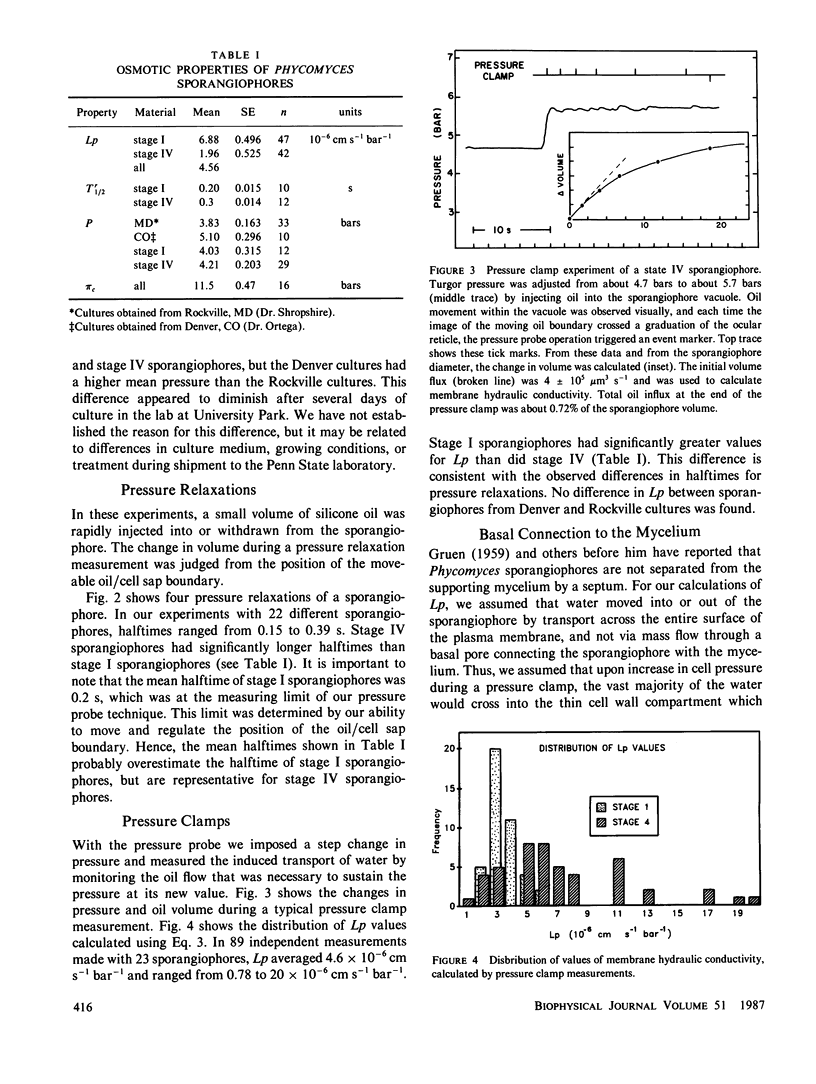

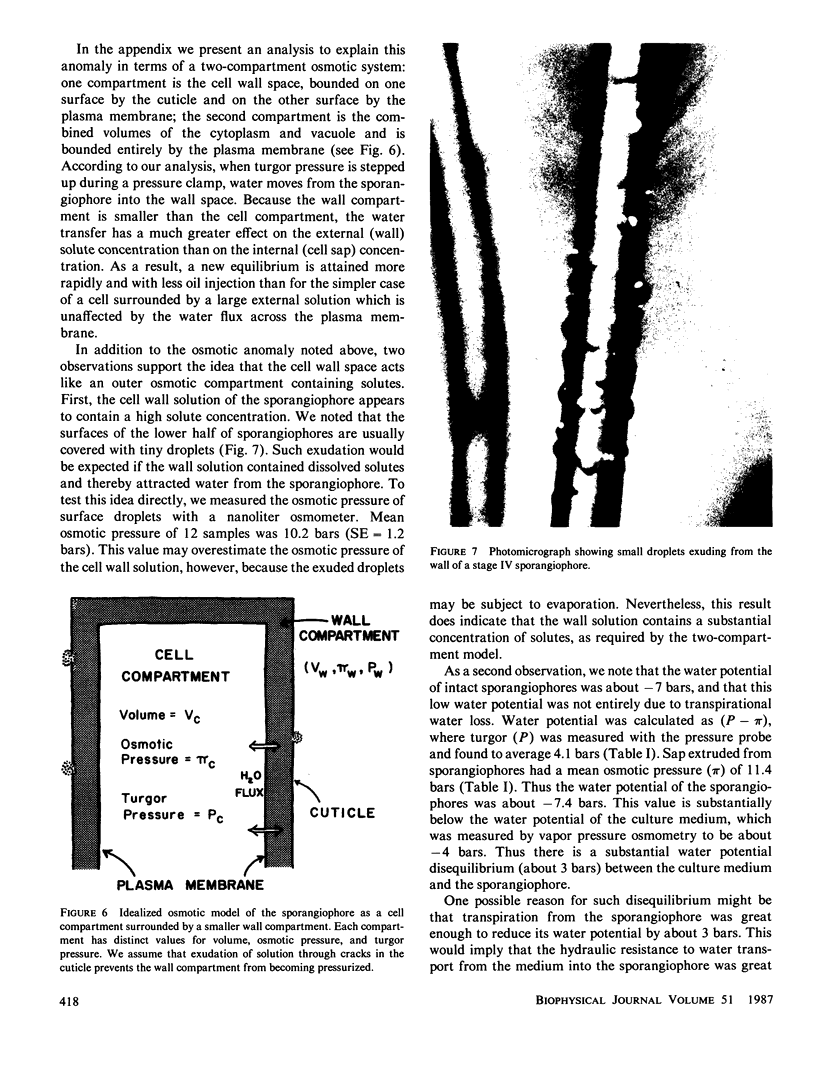




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergman K., Burke P. V., Cerdá-Olmedo E., David C. N., Delbrück M., Foster K. W., Goodell E. W., Heisenberg M., Meissner G., Zalokar M. Phycomyces. Bacteriol Rev. 1969 Mar;33(1):99–157. doi: 10.1128/br.33.1.99-157.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R. J., Jan Y. N., Matricon J., Delbrück M. Avoidance response, house response, and wind responses of the sporangiophore of Phycomyces. J Gen Physiol. 1975 Jul;66(1):67–95. doi: 10.1085/jgp.66.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colotelo N. Fungal exudates. Can J Microbiol. 1978 Oct;24(10):1173–1181. doi: 10.1139/m78-191. [DOI] [PubMed] [Google Scholar]
- Cosgrove D. J., Cleland R. E. Solutes in the free space of growing stem tissues. Plant Physiol. 1983 Jun;72(2):326–331. doi: 10.1104/pp.72.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D. Biophysical control of plant cell growth. Annu Rev Plant Physiol. 1986;37:377–405. doi: 10.1146/annurev.pp.37.060186.002113. [DOI] [PubMed] [Google Scholar]
- Dennison D. S., Shropshire W., Jr The gravireceptor of Phycomyces. Its development following gravity exposure. J Gen Physiol. 1984 Dec;84(6):845–859. doi: 10.1085/jgp.84.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruen H. E. Growth and Development of Isolated Phycomyces Sporangiophores. Plant Physiol. 1959 Mar;34(2):158–168. doi: 10.1104/pp.34.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyure D. C., Bottger B., Gamow R. I. Phycomyces: detailed analysis of the anemogeotropic response. J Gen Physiol. 1984 Nov;84(5):727–738. doi: 10.1085/jgp.84.5.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüsken D., Steudle E., Zimmermann U. Pressure probe technique for measuring water relations of cells in higher plants. Plant Physiol. 1978 Feb;61(2):158–163. doi: 10.1104/pp.61.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendler S., Zimmermann U. A new method for the determination of hydraulic conductivity and cell volume of plant cells by pressure clamp. Plant Physiol. 1982 May;69(5):998–1003. doi: 10.1104/pp.69.5.998. [DOI] [PMC free article] [PubMed] [Google Scholar]